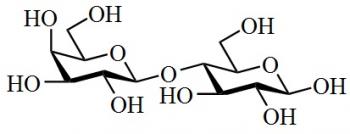
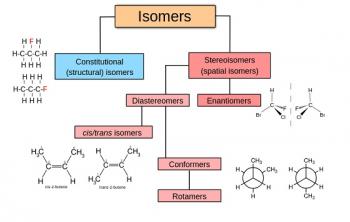
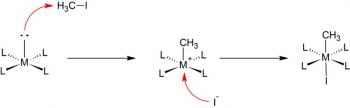
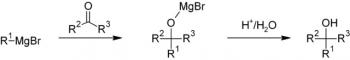
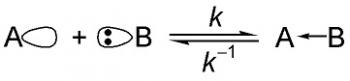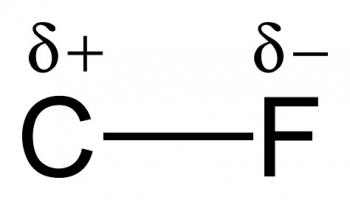Organic Chemistry is the examination of the structures and behaviors of carbon-based compounds. It uses both physical and chemical methods to evaluate these properties. Although it aims to study the behavior of organic substances in its purest form, it also focuses largely on organic reactions within different chemical systems, such as in solutions and mixtures. Even though Organic Chemistry is considered an isolated sub-discipline of chemistry, it is not truly distinct from Inorganic Chemistry, Physical Chemistry or General Chemistry, as it draws on concepts from each of these disciplines.
Organic Chemistry largely focuses on the study of structure, as one of the underlying principles is that structure determines behavior. Thus, by knowing an organic compounds structure, a chemist can predict its behavior under different chemical contexts. A common way to determine structure is through spectroscopy, which is the study of the electromagnetic interactions within the molecule under study. For example, analyzing the different peaks in a mass spectrum will reveal the mass-to-charge ratio and abundance of the different ions that are generated using the mass spectrometer. Thus, using analytical techniques to determine structure, property or reactivity is extremely important in the field of Organic Chemistry.
Since organic compounds form the basis of all life on Earth, the applicability of Organic chemistry is not limited to the laboratory – it also heavily extends into the fields of industry, agriculture, medicine and even botany. Thus, studying Organic Chemistry is essential for the prediction of organic chemical behavior.
© BrainMass Inc. brainmass.com June 30, 2024, 4:42 am ad1c9bdddf