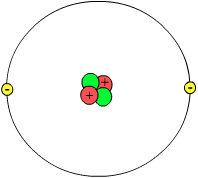
Molecular physics is the study of physical properties of molecules. Atomic physics studies atoms in an isolated system of electrons and the atomic nucleus. Both of these fields of physics are concerned with electronic structures and the dynamic processes by which the arrangements change. These fields are subsets of the study of quantum mechanics.
Molecular physics takes a quantum chemistry approach in order to solve complex problems. It takes the atomic orbital theory and applies it to the molecular orbital theory. Molecular physics looks at the atomic processes that occur in molecules and the effects on the molecular structure. Molecular physics also looks at excited molecules, the quantized vibrations and the discrete energy levels. It is important in various types of spectroscopy experiments. Molecular physics research overlaps with theoretical chemistry, physical chemistry and chemical physics.
Atomic physics primarily looks at the arrangement of electrons around the nucleus and how these electron arrangements change. It also looks at ions and neutral atoms arrangements. Although atomic physics is often associated with nuclear physics, these fields of study are different. Nuclear physic is the field which studies atomic nuclei alone. Atomic physics is a larger branch of molecular and optical physics.
Since the invention of the computer, atomic and molecular physics has advanced at a rapid pace. Computational analysis allows for larger and more sophisticated models to be studied. Computers have also advanced the technologies of accelerators, detectors, lasers and magnetic field generators to assist in experimental work.
© BrainMass Inc. brainmass.com June 30, 2024, 4:31 am ad1c9bdddf