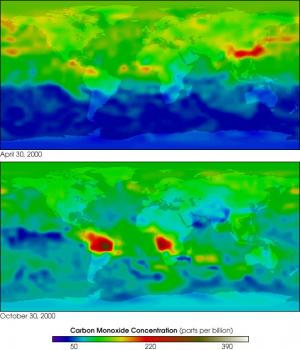
Environmental Chemistry is the examination of biochemical phenomena that occur in natural environments. Its primary focus is on the properties of certain chemicals found in the air, soil and water. Although it studies how the uncontaminated environment works, by analyzing aspects such as natural concentrations of different chemical substances, it also takes into account the effects of human activity in each of these environments.
Important concepts are drawn from other Chemistry disciplines, such as chemical reactions, solubility and different analytical techniques. These concepts are then applied to specific environmental topics, such as contaminants, radiochemicals and environmental indicators, to better understand the underlying chemistry behind these phenomena.
For example, there are many chemical indicators which can be measured independently or in conjunction to assess the quality of water. The level of oxygenation, or the pH, or both of these measurements can be measured to assess whether for example, the water in a certain lake is suitable for habitation of a certain species, or whether it is in fact hazardous and sub-optimal.
Environmental Chemistry is becoming increasingly important as many Environmental Agencies use its concepts and techniques for the detection and identification of pollutants, such as heavy metal contamination from nearby industries, or urban runoff from a nearby city. Thus, although many of the concepts are drawn from different Chemistry disciplines, it focuses more on the properties of chemicals in its natural context.
© BrainMass Inc. brainmass.com June 30, 2024, 10:04 am ad1c9bdddf