General Chemistry is the theoretical study of fundamental physical principles as they apply to chemical phenomena. It examines the applicability of these principles on a broad spectrum of topics which include atomic and molecular structure, bonding and modern quantum theory. Thus, General Chemistry serves not only as an introduction, but also an essential cornerstone for other chemistry disciplines.
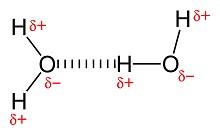
General Chemistry explores the basic rules and properties of atoms, which are the basic units of matter. The primary challenge of studying General Chemistry is knowing when to apply the different rules and properties within varying chemical contexts. Such understanding can help predict how a particular atom will react under a particular condition, and even the eventual outcome of different chemical reactions. In addition to individual atomic properties, General Chemistry also aims to examine how atoms interact with each other, whether they attract or repel, and the conditions which permit the formations of different bonds. Thus, this knowledge is essential for successful theoretical prediction of atomic behavior.
General Chemistry is not limited to atomic theory, as it also explores modern quantum theory in different chemical systems. Entities smaller than atoms are considered and their properties in these sub-atomic levels can be examined, in addition to atomic behavior, to help predict chemical phenomena in the different branches of chemistry.
© BrainMass Inc. brainmass.com June 30, 2024, 10:05 am ad1c9bdddf