Inorganic Chemistry
BrainMass Categories within Inorganic Chemistry
Inorganic Chemical Reactions
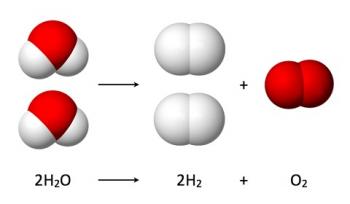
Inorganic Chemical Reactions can be categorized into the following broad categories: combination reactions, decomposition reactions, single displacement reactions and double displacement reactions.
The Periodic Table
The Periodic Table is the arrangement of elements in tabular form based on atomic numbers and electronic configurations.
BrainMass Solutions Available for Instant Download
Oxidation State and Isomers in Octahedral Complex
For questions 5-7, determine the oxidation state of the metal in each of the following complexes. Draw ALL linkage isomers and stereoisomers for each complex CLEARLY showing enantiomers. Where possible, label each isomer as cis, trans, fac, mer, Δ and/or Λ. You must properly use shorthand notation for multidentate ligands.
Octahedral Complex: oxidation state, isomers and CFSE
1. Determine is the oxidation state of the metal in each of the following complexes. Draw ALL linkage isomers and stereoisomers for each complex CLEARLY showing enantiomers. Where possible, label each isomer as cis, trans, fac, mer, and/or . You must properly use shorthand notation for multidentate ligands. Note: do
Symmetry adapted linear combinations
Use this example for the following two problems on page 2: l) Projection of the fx(x2-3y2) orbital. Assume the lobes of the orbital are coplanar and the z axis is perpendicular to the page. 1. Consider an atom having the fx(x2-3y2) orbital projection presented above. What d and p orbitals of a second atom would h
Point group questions
1. Determine the point group for each of the following molecules and items. Draw a clear picture of each molecule or item and show or clearly describe ALL the symmetry elements necessary to determine the point group. NOTE: Only the connectivity of the atoms determines symmetry, not the bond order between atoms. a) phos
Chemistry Point Groups
For questions 1-5 below, determine the point group for each of the following molecules, ions and objects. Draw a clear picture of each molecule, ion and object and clearly show or describe all symmetry elements necessary to determine the point group. Remember, only the connectivity of the atoms determines the symmetry, not the
Possible cation anion rates for different molecular arrangements
The question is about the mathematical constrain of the possible cation anion ratios for cubic, octahedral and tetrahedral arrangements. The proof is in the attachment along with the additional questions. http://aerostudents.com/files/materialsAndStructures/MaterialsScienceBookSolutions/CH12.pdf
Applications of Inorganic Nanoparticles
I need some information regarding the uses and applications of inorganic nanoparticles. Please use published articles and cite when needed.
Types of Chemical Reactions
This solution will discuss the 6 major groups of chemical reactions and provide an example and the general formula of each type.
chemQ
1. Combustion of 5.13g of ibuprofen a widely used painkiller produces 14.224g CO2, 4.029g H2O. Ibuprofen contains only carbon hydrogen and oxygen atoms. If the molecular weight of ibuprofen is liss than 400g/mol determine the molecular formula. 2. A. From the data on the last page calculate the free energy change for the rea
Inorganic/Analytical - Dilution in Titration and Water Hardness
2. What is the effect of reagent and analyte dilution on the equivalence point pH in strong acid-base titration? 3. You collect a water sample from your shower containing 1.2 mM Mg^2+ +3.3 mM Ca2+. (FM for CaCO3=100.09)
EDTA Determinination of Metals
A mixture of Mn2+, Mg2+ and Zn2+ was analyzed as follows: The 25000 mL sample was treated with 0.25 g of NH3OH+Cl- (hydroxylammonium cgloride, a reducing agent that maintains manganese in the +2 state), 10 mL of ammonia buffer (pH 10), and a few drops of erichrome black T indicator and then duluted to 100 mL. It was warmed to 40
EDTA-pY3+ Titration Plot
Consider the titration of 50.0 mL of 0.0110 M Y^3+ (y=yttrium) with 0.0220 M EDTA at pH 5.00. Calculate pY^3+ at the following volumes of added EDTA and sketch the titration curve: (a) 0mL (b) 10.0 mL (c) 25 mL (d) 30.0 mL.
Calculation of percentage yield of nitrone
Materials and Methods Protocol was titled Synthesis and application of a radical trapping agent was obtained from CHEM 3880-A1. For the synthesis of N-Benzylidene-tert-butylamine N-Oxide (2) in a 50 mL Erlenmeyer flask along with a flea sized stir bar 0.91 mL of N-tert-buyl benzylamine , 0.07 g of Na2 WO42H2O and 10 mL of
Ligand Substitution of Coordination Complexes
I am confused on how to approach these problems. This is an ungraded class review and I would like to understand before going to class, so I can ask additional questions. Thanks for the assistance. 2. Is a high spin or low spin d6 metal ion complex most likely to undergo substitution by a dissociative process? Explain the rea
Strong Pi Donors and Acceptors
I need help with this question: 1. Six-coordinate CR(3) complexes of the type trans-(CrL4A2)n+, generally have magnetic moments consistent with three unpaired electrons, which suggests occupancy of the d orbitals as shown to the right. In principle, a complex with only one unpaired electron could be generated by a suitable choi
Isomers and Ligands
4. Circle the letter corresponding to the correct answer for the following. For which of the following complexes can a tetralredral coordination geometry be unequivocally excluded based upon its magnetic properties? a) Cu(PPh3)3Cl (diamagnetic) b) P(PPhr3)2Cl2 (diamagnetic) c) Ni(PPh3)2Br2 (paramagnetic) d) Co(PPh3)2Cl2 (par
Lattice Energy and LFSE
See the attached file. 3. A plot of the lattice energies for MCI; salts for M = Ca to Zn and a drawing of the cell are shown to the right. In the electrostatic model for "ionic bonding" U is proportional to (Z+)(Z-)e2A/(r+ + r.) where Z+ and Z- are the ion charges, A is the structure (Madelung) constant and r+ + r are the radi
Hydroformylation Zeroing Results
Sir, I have located the articles and maybe one more. I am having trouble zeroing in on the right answer for the questions and format (like should I put it in a table) since formatting/clarity is graded. Also, how do I go about finding the articles. When I queried on the [Rh(PPh3)3(H)(CO)] or hydroformylation or whatever, I g
Point Group Symmetry of a Coordination Complex
I am confused and frustrated on this topic. I do not know how to begin or exactly what is being asked. An explanation with work would be helpful. A question like this will appear on our next quiz. Thanks.
MO Diagram: Symmetry and Bonding
I have attached a question involving bonding and symmetry. I am totally confused on how to do this. Any explanation with work would be greatly appreciated. I am going to have similar problem on the next exam.
NMR Spectra of a Product
Obtain a 1H NMR spectra of the product in CDCl3 (given with this posting). Integrate the two low-field sets of peaks at 3.8 and 4.2 ppm, analyze the coupling constant (J) patterns to assign the two sets of peaks (as I discussed before, the MNova software auto assigns it for you), and report the percentage distribution of cis- an
Diastereoisomers of 3,3,5-trimethylcyclohexanol
I have attached the data in pdf form (GC information); document that is the peaks for NMR integrated (chloroform as the deuterated solvent) and a word doc in rich text (duplicating the excel format) that must be completed. I need assistance with calculations.
GC of the diastereoisomers of 3,3,5-trimethylcyclohexanol
I need the cis and trans alcohol % from the attached information received by GC. The solvent was methanol. I believe this is done by using the peak area but I'm not sure about how to go about it.
Finding the NMR of the Diastereoisomers
I need help finding the cis and trans alcohol % of the H'NMR from the attachment. The deuterated solvent was chlorform. I have rerun with auto integration but don't know to find j value, I am new the software MestReNova.
Molecular Orbitals and Walsh Diagram: BeH2
I have attached a four part question concerning bond lengths. I am confused on the topic and we will have a question similar to this one on our next exam. I would like to increase my understanding before the exam. Please include as much information and assistance as possible. Thank you for the assistance.
Precipitation formation test
Question #1: If 200 mL of 0.300 M Cr(NO3)3(aq) is added to 100 mL of 4.0 * 10^-4 M NaF(aq) at 25 degrees celcius, will a CrF3 precipitate form? For CrF3 in water at 25°C, Ksp = 6.6 * 10^-11. Question #2: The 3dxy orbital has its high probability regions lying between the x and y axes. The 3dz2 orbital has its high probabili
Cobalamin complex
I have my final report and I have 2 questions about it. So our final product was a brown crystalline solid that had an absorbance at about 880nm. My first question is how is brown reflected (not in a literal sense) in the visible spectrum? that's outside visible region and I could find nothing about brown solids and absorbance
Chelate with a metal ion
In forming a chelate with a metal ion, a mixture of free EDTA (abbreviated Y^4-) and metal chelate (abbreviated MY^n-4) can buffer the free metal ion concentration at values near the dissociation constant of the metal chelate, just as a weak acid and a salt can buffer the hydrogen ion concentration at values near the acid dissoc
Electron Configurations of Atoms
What is wrong with the following electron configurations for atoms in their ground states? a. 1s^(2)*2s^(2)*3s^(1) b. [Ne]2s^(2)*2p^(3) c. [Ne]3s^(2)3d^(5) Please provide a detailed explanation. Thanks!
Symmetry Labels of Vibrational Modes of a Molecule
The vibrational modes of a molecule are assigned symmetry labels that tell us something about the symmetry of the vibrational mode. Given the symmetry labels below, specify what each part of the symbol means. a) E_2g b) (A_1)'
