The Periodic Table
BrainMass Categories within The Periodic Table
Periodicity
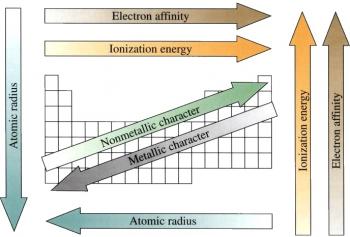
Periodicity refers to the recurring trends seen among the elements of the periodic table.
Halogens
Halogens refer to Group 17 in the periodic table consisting of the elements: fluorine (F), chlorine (Cl), Bromine (Br), Iodine (I) and Astatine (At).
Gases
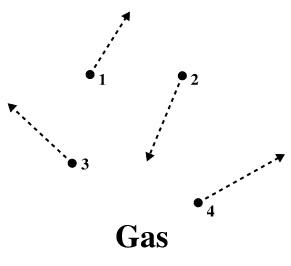
Gas is one of the four fundamental states of matter found between the liquid and plasma states, where their particles are widely separated and have very weak intermolecular bonds between the atoms.
Transition Metals

Transition Metals refer to any elements found in the d-block of the periodic table.
BrainMass Solutions Available for Instant Download
Atomic Size and Electronic Structure
1. What happens to the atomic radii as you go down a column in the periodic table,e.g. from Li to Fr? 2. Why does the trend you identified in question 1 occur? Be sure to refer to electronic structure in your answer 3. What happens to the atomic radii as you go across a row in the periodic table, e.g. from Li to Ne?
Density and Melting Point
Questions on density: 1. Using the table below determine any trends of density that might be related to the periodic table. Substance Density (g/L) Substance Density (g/L) H2 0.0899 O2 1.429 He 0.1785 N2 1.2506 Ne 0.9
Reactivity of Metals
1. What is the order of activity of the metals in your study? (Arrange them from most reactive to least reactive). Consider the metals used in demonstration - sodium, potassium and calcium in addition to the ones you used in experiment - copper, magnesium, zinc. 2. Why is calcium a poor choice for a metal to be used in pi
Representative Elements
The 'representative elements' are those in the first two columns and the last six of the periodic table. The middle region of the table represents the transition elements. When chemists discuss the periodic nature of the elements they focus on mainly the 'representative elements'. Find two websites that discuss the Perio
Paramagnetic
What elements are paramagnetic in the second row of the periodic table?
Periodic Table
Nearly every compound of silicon has this element in the +4 oxidation state. In contrast, most compounds of lead have this element in the +2 oxidation state. a) What general trend of the oxidation states does this observation illustrate? b) What could you suggest as an explanation for this observation?
Periodic Table - Observations
Explain the following observations. a) In2O is more basic than In2O3 b) Pi bonds exist between two nitrogen atoms but not between two phosphorus atoms c) Lithium salts are more readily soluble in polar organic solvents (ethanol, acetone) than in water
Periodic Table - Naming & Formulae
Give the name and formula for the following: 1. Synthetic abrasive composed entirely of group 14 elements: (From Group 14) 2. Compound used in solution in the photographic process: (From Group 16) 3. Compound used in etching glass: (Group 17)
Periodic Table
Nearly every compound of silicon has this element in the +4 oxidation state. In contrast, most compounds of lead have this element in the +2 oxidation state. a) What general trend of the oxidation states does this observation illustrate? b) What could you suggest as an explanation for this observation?
Understanding the arrangement of elements within the periodic table. Determining the number of electrons in the outermost shell.
Three elements are listed below. How many electrons does each have in its outermost electron shell? (1) aluminum (2) krypton (3) potassium
