Physical Chemistry
BrainMass Categories within Physical Chemistry
Gas Laws
Gas Laws describe the behavior of gases through examining the relationships between their physical properties.
Acids and Bases

A base is a compound that can donate an electron pair, while an acid is a compound that can accept an electron pair.
Oxidation-Reduction and Electrochemistry
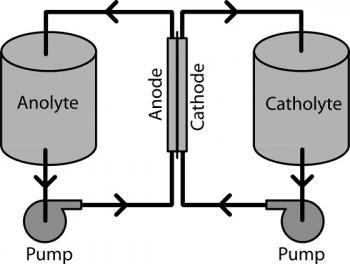
Electrochemistry refers to a set of chemical reactions which take place in solution between an electrode and an electrolyte.
Chemical Kinetics
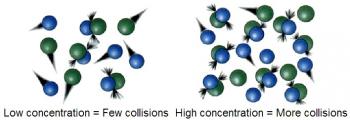
Chemical kinetics is the study of the rates of chemical reactions.
Chemical Equilibrium
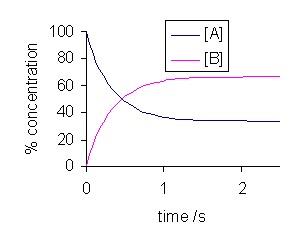
Chemical Equilibrium is the state in which the forward rate of a reaction equals its reverse rate of reaction.
Energetics and Thermodynamics
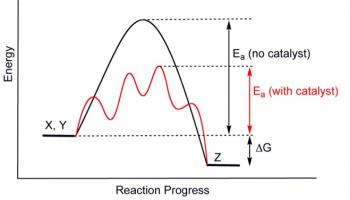
Thermodynamics is a branch of Energetics concerned with the concepts of heat, energy and work.
Stoichiometry
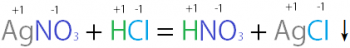
Stoichiometry refers to the study of relative quantities of reactants and/or products within a defined chemical system.
Materials Chemistry
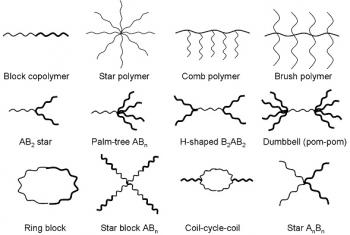
Materials Chemistry is the examination of chemical synthesis and its application in the discovery of new materials.
BrainMass Solutions Available for Instant Download
Work and Energy for a Heat System
Please work the following practice problem. Being very thorough. Explain how you intend to approach the problem, important information and or equations that are relevant to solve/understand the thought process. Consider a situation in which the surroundings heat a system and the system contains a gas which expands against a
Mathematical Relations for Coefficient of Performance
This page shows efficiency of a carnot engine. http://www.oberlin.edu/physics/dstyer/P111/Carnot.pdf The refrigerator uses the same process only it run backwards. By using the same recipe as in the link above I want a mathematical derivation of the coefficient of performance of a refrigerator. A little additional informati
Concept of Partial Derivative and Thermodynamic Derivations
Question is attached. It is about illustrating a mathematical relation that has both partial derivatives with p and T constant in a graph. And also about the derivation for the joule-Thomson effect.
Boltzmann Distribution Problem about Defining Energy
The question is about understanding how they define the energy levels in a derivation from Wikipedia. It is attached with a link to the internet site.
Calculation involving a change in blood pH.
Although the pH of human blood is normally between pH 7.3-7.5, certain situations can take place which bring about "acidosis." Acidosis is defined as an abnormal lowering in the blood pH. If the decrease in pH is severe, the individual may go into a coma or possibly even die. Calculate the increase in H+ concentration corresp
Physical Science: Temperature, Climate, and Renewable Resources
1. Explain how the temperature difference between the poles and the equator drives the motion of the oceans and atmosphere. 2. What are the differences between ice caps and glaciers? 3. How do ocean currents affect local climate? 4. Why do we call groundwater in most areas a "nonrenewable resource"? 5. What is the Gulf Strea
1.0 M solution of a strong acid, HA
Are the following statements about a 1.0 M solution of a strong acid, HA, true or false? [A-] › [H+]: True/False The pH is 0.00: True/False [H+] = 1.0 M: True/False [HA] = 1.0 M: True/False From the given solubility data given, calculate the value of Ksp for the following compounds. SrF2; 7.3 x 10-2 g/L Ag3PO4; 6.7 x
Calculating Pressures using different laws
Converting between Units of Pressure Part A: Convert 0.700atm of pressure to its equivalent in millimeters of mercury. Express the pressure numerically in millimeters of mercury Part B The Pressure in car tires is often measured in pounds per square inch (lb/in2) with the recommended pressure being in the range of 25 to
Partition Coefficient in Octanol
A monoprotic acid with a Ka of 2.83 x 10-5 has a partition coefficient of 2.8 (favoring octanol) when distributed between water and octanol. Find the formal concentration of the acid in each phase when 100 mL of 0.10 M aqueous acid is extracted with 37 mL of octanol at pH 8.00.
Order of a reaction
Consider a kinetic study of oxidation of a new plastic stabiliser being proposed for the automobile industry. Stabiliser + H+ + O2 ----> Byproduct Trial [ Stabiliser] [H+] [O2] Rate (M min^-1) 1 0.40 0.30 0.560 7.14 x 10^ -4 2 1.20 0.30
Calculating Volume Needed for Level of pH
A 1.00g sample of benzoic acid (C6H5CO2H, FW = 122.12g/mol,Ka = 6.5 x 10^ -5) is dissolved in water to give a solution with a volume of 500mL. What volume of 2.13M aqueous sodium benzoate (NaC6H5CO2) must be added to the aqueous benzoic acid solution to obtain a buffer with pH = 5.00?
pH and Percent Ionization
1. Determine the pH and the percent ionization in a 0.50M solution of KOBr. The Ka for HOBr = 2.5 x 10 ^ -9. 2. Calculate the number of grams of KOBr that must be added to 1.5L of 0.200M HOBr to prepare a buffer solution of pH 8.50. Assume no change in volume. The Ka for HOBr = 2.5 x 10^-9.
PCl5 in a heated glass bulb
a) A weighted amount of PCl5 is sealed in a 100.0mL glass bulb and heated to 250'C. When the system reaches equilibrium, the pressure in the bulb is 0.895atm. If Kp = 2.15 at 250'C, what is the partial pressure of each of the three gases at equilibrium? PCL5(g) ===========> PCl3(g) + Cl2(g) b) Calculate Kc at 250'C.
Calculating Delta H
Use some or all of the following reaction enthalpies to calculate delta H for the reaction: 3NH3(aq) + ClO- (aq) --> N2H4(g) + NH4+ (aq) + Cl- (aq) + OH- (aq) Cl- (aq) + H2O2(l) --> H2O(l) + ClO- (aq) delta H = - 6.5kJ/mol N2(g) + 3H2(g)--> 2NH3(g) delta H = -160.6kJ/mol H2O(l) + 1/2 N
Kc for reactions
Write the expressions for Kc for the following reactions. a. 2O3(g) --> 3O2(g) b. Ti(s) + 2Cl2(g) --> TiCl4(l) c. 2C2H4(g) + 2H2(g) --> 2C2H6(g) + O2(g) d. C(s) + 2H2(g) --> CH4(g) e. 4HCl(aq) + O2(g) --> 2H2O(l) + 2Cl2(g)
Nernst Equation
You are titrating 100.0 mL of 0.0400 M Fe^2+ in 1 M HClO_4 to give Fe^3+ and Ce^3+ using Pt and calomel electrodes to find the endpoint. a) Write the balanced titration reaction. Ce^4++Fe^2+ Ce^3++Fe^3+ Complete the two half reactions for the Pt electrode. Ce^4+ + e^- Ce^3+ + e^-
Voltiac (Galvanic) Cells
Please see attached file and answer the question involving titations in a voltiac cell.
Standard potentials measured against the standard hydrogen electrode
Standard potentials are measured against the standard hydrogen electrode (S.H.E.). Because it is not always convenient to use a S.H.E., often other reference electrodes are used. The saturated calomel electrode (S.C.E.) is one commonly used reference electrode, with a potential of 0.242 V versus the S.H.E. Using a table of stand
Molar Mass of Sulfur Based on Molality
What would the molar mass of sulfur be based on the following data and information? 2.07 grams of sulfur ( solute ) and 22.91 grams of naphthalene (solvent) was used. The Kf of naphthalene is 6.9 oC/m and delta T = Kf x m . The melting point of naphthalene was 80.50 oC and the melting point of the mixture was 78.0 o C. Re
Conditional Formation Constant - EDTA
Question 1: a) How many millimeters of 0.0700 M EDTA are required to react with 50.0 mL of 0.0140 M Cu2+? b) With 50.0 mL of 0.0140 M Sc3+? Question 2: a) Find the conditional formation constant for Sr(EDTA)2- at pH 11.00, where log Kf is 8.72 and αY4- is 0.81. = _________ b) Find the concentration of free
Change in Internal Energy: Example Problem
a) Identify the following systems as open, closed, or isolated. i) Coolant in a refrigerator coil ii) The combustion of liquid hexane in a bomb calorimeter b) A gas sample heated in a cylinder uses 650 kJ of heat. A piston that compresses this gas does 800 kJ of work. what is the change in internal energy, delta E, of the
Determining System Equilibrium
For the reaction 2HI(g) <=====> H2(g) + I 2(g) at 458'C, Kc is 2.59 x 10 -2 A one-litre cylinder at 458'C is found to contain 0.36 moles of HI (g), 0.15 moles of I 2(g), and 0.18 moles of H2(g). Is this system at equilibrium?
Equilibrium concentration for the reaction
the equilibrium constant for the reaction N2O4(g) <=======> 2NO2(g) is Kc = 4.66 x 10 -3 at 298K. Calculate the following when 0.800mol of N2O4(g) is injected into a closed 1.00L container. a). The concentration of each gas once equilibrium is reached. b). The new equilibrium concentrations if
Find Delta U, Delta H
I have review problems for an upcoming exam and what to make sure I know how to do these problems. I have some idea and some work but I would appreciate conformation. Please include work and explanation. Thank you for the assistance. See the attached file. 2. The virial equation of state for NH3 (g) can be written as Us
Ideal Gases and Final Temperatures
1. Given that (dU/dV)T = 0 for an ideal gas, prove that (dH/dV)T = 0 for an ideal gas. 2. Calculate the final temperature, the work done, and the change of internal energy when 0.200 mol NH3 is used in a reversible adiabatic expansion form 0.50 dm3 to 2.00 dm3, initially at 25oC. The molar heat capacity of NH3 is 27.0 JK-1mol-1
Concentration of a Species
One mole of NOCl gas is added to a 4.0L container. The NOCl undergoes slight decomposition to form NO gas and Cl2 gas. If the equilibrium constant, Kc, for the reaction is 2.0x 10-10 at 25'C, calculate the concentration of all the species at equilibrium at this temp.
Differences in Boiling Points
Rationalize the difference in boiling point between the members of the following pairs of substances: HF(20oC) and HCl (-85oC) CHCl3 (61oC) and CHBr3 (150oC) Br2 (59oC) and ICl (97oC) Please provide detailed explanation.
Physical Propoerties and Intermolecular Forces
What type of intermolecular forces accounts for the following differences in each case? CH3OH boils at 65oC, but CH3SH boils at 6oC. Xe is a liquid at atmospheric pressure and 120 K, whereas Ar is a gas. Kr, atomic weight 84, boils at 120.9 K, whereas Cl2, MW ? 71, boils at 238 K. Acetone boils at 56oC, whereas 2-methylpropa
Molar Mass from Volumetric Data
2.64g of an organic base, isolated from a shrub in the mountains of Peru was dissolved in 100mL of water. 25.0mL of this solution required 27.5mL of 0.250mL HCL for complete neutralization. It was later determined that each molecule of the unknown base could accept two protons. What is the molar mass of this unknown compound?
Concentration of Pb2+ in 0.0010M KI Saturated with Pbl_2
Formatted version is attached. -------------------------- Find the concentration of Pb2+ in 0.0010M KI saturated with Pbl_2. Include activity coefficients in your solubility-product expression. The K_sp of Pbl_2 os 9.8 x 10^-9. [Pb^2+] = _________M Hint from the program: K_sp=[Pb^(2+)] γ_(Pb^(2+))[I^-]^2 γ^2_(I^-) A
