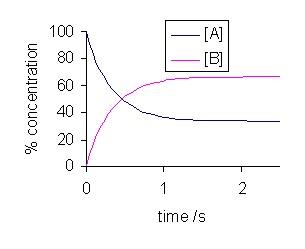
Gas Laws describe the behavior of gases through examining the relationships between their physical properties. Despite the wide differences in chemical properties of all gases, they more or less obey the gas laws. For example, although both H2 and O2 are very different gases, with molecular weights of 2 g/mol and 32 g/mol respectively, they will behave similarly to a change in any physical property.
Gas is one of the four fundamental states of matter, found between the liquid and plasma states, where their particles are widely separated and have very weak intermolecular bonds between the atoms. Gases are the only states of matter that can be compressed or even expand to fill a very large space. It is these properties which makes them subject to behave differently with respect to changing physical properties such as pressure, volume, temperature and amount.
The main gas laws include Avogadro’s Law, Boyle’s Law, Charles’ Law, Fick’s Law of Diffusion, Gay-Lussac’s Law and Henry’s Law. All of these examining the relationship between physical properties to help predict the overall behavior of that gas. Thus, understanding the gas laws and when to apply them is crucial to help predict how a gas will react to varying conditions.
© BrainMass Inc. brainmass.com June 30, 2024, 10:04 am ad1c9bdddf