Experimental Design and Methods in Chemistry
BrainMass Categories within Experimental Design and Methods in Chemistry
Chromatography
Chromatography refers to a set of physical techniques used for separating or analyzing mixtures.
Titration
Titration is a quantitative analytical procedure used to determine the unknown concentration of an identified pure chemical substance.
Qualitative Analysis
Qualitative Analysis is a method used primarily for the detection of a particular compound in a sample substance, without measuring values related to quantity.
Spectrum Analysis
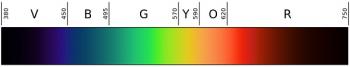
Spectrum Analysis is the study of electromagnetic interactions of matter in order to determine its chemical and physical properties.
Quantitative Analysis
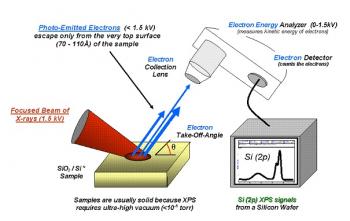
Quantitative Analysis is a method used for the determination of amounts and proportions regarding a sample substance.
BrainMass Solutions Available for Instant Download
Spectrophotometric Measurements
What are the advantages and disadvantages in using spectrophotometric measurements for the analysis of various compounds? Explain Beer's and Lambert's laws and how are these important to spectrophotometric measurements? How are spectrophotometric measurements used for the determinations of content of phenolic compounds in
Methods of Extracting Bioactive Phytochemicals from Fruit
Please list methods for extracting bioactive phytochemicals from fruit and their difference and characteristics.
Chromatography
How do you determine an instrument's (hplc or gc) detection sensitivity? How do you determine the detector's limit of detection (LOD) and limit of quantitation (LOQ)?
Gas Chromotography
One of my biggest problem in answering the discussion, statistical analysis, significance and conclusion is I have trouble determining what the results really mean. Would you please help me interpret my results, such as what did the experiment demonstrate and how do I know? Like in the last one I knew to discuss time and peaks.
Entropy change for isothermal process
1. Given that the Gibbs function is: G=H-TS (H=U+PV dU=TdS-pdV etc.) And since the Gibbs function is a "state function" and forms exact differential, find a simple expression for the change of entropy with respect to pressure for an isothermal process with an ideal gas:
GC-FPD: Analyzing A Chemical Analyte
Choose any type of chemical analyte in a food product and please describe in detail how to anlayze the anlayte using a GC-FPD. please include sample preparation and extraction and indicated specific what is mobile phase, column, and detector which used.
Calculating Time of a Cyclotron's Activity Production
Calculate how long will it take to run the cyclotron to produce an activity of 18^F at 75% of its saturation value. If it takes 30 minutes to filter and conjugate deoxyglucose to produce 18^FDG, what is its activity at the time of PET imaging? The proton beam current is 40 μA. The proton beam impinging onto the H2^18 target (10
Description of the Chromatography Technique
What is Chromatography? How do differences in polarity and molecular weight between amino acids affect migration in a mixture of proteins?
Experimental Sources of Error
You used 25.0 ml of KI, 48.0 ml of KNO3, 25.0 ml of( NH4)2S2O8 along with 1.0 ml of Na2S2O3 to go along with 1.0 ml samples of Na2S2O3 after each color change. Which of the solutions listed will have the biggest source of error in your readings if the solution is off by even 0.1 ml? Please provide detailed explanation. Thank
Electrostatic Energy of Non-Ionized Atoms
When we model proteins on the computer, we must assign a partial charge to each of the non-ionized atoms. Let's consider the hydrogen bond between a carbonyl oxygen with a partial charge of -0.5 and an amino hydrogen with a partial charge of +0.3. The distance between these atoms is typically about 2A. Calculate the electrost
Accuracy of Balances
The balance of mettles was 100.01g and sartorius was 100.01. Would you assume that since a balance reads 0.01g, it's weight is actually 0.01g?
Find ppm of sodium based on cation-exchange
Reagents: .02 M NaOH, 1M HCl, Bio-Rad Dowex 50W-X2 (100/200 mesh) cation-exchange resin, Unknown Saline Solution diluted to the mark given in a volumetric flask. Procedure: 1. Prepare the resin column using Bio-Rad Dowex 50W resin. 2. While trying to minimize resin disturbance, add ~ 10 mL of 1 M HCl slowly into each col
Benedict's Test.
The Managing Director of a well-known company on Wall Street thrives on a diet of fruit jam, bread, pasta, and coffee. She exercises intermittently. One day she decides to go to her primary healthcare provider for a routine checkup. The healthcare provider recommends that she take the Benedict's test. Assume that the glucose lev
Determination of Ca2+ and Mg2+ with EDTA
Procedure: 1-It is important to use good deionized water for this procedure-traces of heavy metal ions will form irreversible complexes with calmagite and interfere with end point. 2-Weigh 3.0 g NaH2Y. H2O , transfer to 1L plastic bottle and dissolve in about 500 mL water. Add 15 mL 1% Mg Cl2 solution and 2 mL 6M NH3, Mix tho
4 Analytical Chemistry Questions.
1. If you wish to extract aqueous acetic acid into hexane, is it more effective to adjust the aqueous phase to pH 3 or pH 8? 2. A chromatogram with ideal Gaussian bands has tr = 17.5 min and w 1/2 = 1.50 min. A) How many theoretical plates are present? B) Find the plate height if the column is 10 cm long. 3. Match t
How to create serial dilutions from a stock solution.
2,3,7,8-Tetrachloro-dibenzo-p-dioxin (TCDD) is pre-made at 32pg/ul = 100nM. The total volume is 200 uL. I need to prepare a working solution of this compound at 30nM per 1mL, then 3nM per 1mL, and finally at 0.3nM per 1mL.
Benedict's Test and Ketohexose
The Managing Director of a well-known company on Wall Street thrives on a diet of fruit jam, bread, pasta, and coffee. She exercises intermittently. One day she decides to go to her primary health-care provider for a routine checkup. The health-care provider recommends that she take the Benedict's test. Assume that the glucose l
You are asked to dilute a vial (1g) of powder-c to 330mg/ml concentration. The patient needs a 600 mg dose. Label reads dilute with 2.5ml normal saline.
1. You are asked to dilute a vial (1g) of powder-c to 330mg/ml concentration. The patient needs a 600 mg dose. Label reads dilute with 2.5ml normal saline. a. How much dilute was added? b. How much was withdrawn for the dose? c. What size syringe was used? 2. A patient needs 14mg of hazardous powder. Two vials of 10mg
amines in hnmr and cnmr
An example of an amine containing drug is Procaine which contains 2 amine groups.When HCl is added to procaine I think the tertiary amine is protonated as it has more electrons and the benzene amine is delocalised by resonance with the ring so is less available for proton bonding but how can I distinguish between the protonated
Determination of Chloride by Precipitation Titration
DETERMINATION OF CHLORIDE BY PRECIPITATION TITRATION Abstract: Hydrogen Peroxide (H2O2) solution was titrated with a standardized potassium permanganate (KMnO4) solution and it was found that the unknown solution contained 11.3% (wt/vol%) H2O2. were titrated using silver ions as a titrant. These titrations were first used
Argentometric titrations
Argentometric titrations typically use a standard solution of silver ions to form a silver halide precipitate. Calculate the pAg+ value when 25.0 mL of 0.100 M AgNO3 is added to 25.0 mL of 0.0500 M chloride ion. A solution of NaIO3 was used to titrate 50.0 mL of a mixture containing 0.100 M Ca2+ and 0.100 M Ba2+. Calculate th
Zinc in a Cough Drop
I just did a lab that dealt with finding the amount of Zinc found in cough drops. I did this by EDTA titration. First I dissolve one cough drop in 50mL's of a acetate buffer in an erlenmeyer flask. Then once dissolved I titrated it with .013M of EDTA solutiuon until its endpoint. My initial reading on the buret was 23.4mL of EDT
Simple Chemistry Questions
1. Describe how you would prepare 1 liter of each of the following solutions. a). 1.5 M glycine b). 0.5 mM glucose c). 10 mM ethanol d). 10 mM hemoglobin 2. Describe how you would prepare just 100ml of each of the solutions in problem #1 above 3. When preparing a solution, why do you dissolve the component in less d
Science
Change is influenced by many different factors in many different situations. If you imagine the 400,000 gallons of water in a stream at the top of a hill, you can picture that they will flow down the hill in order to reach equilibrium. But what if the temperature outside is -40 degrees Fahrenheit? How might this change the movem
Haloalkanes, Anaesthetics and Tests for Sugars
1. Identify a haloalkane and describe the manner in which it functions as an anesthetic. 2. The Managing Director of a well-known company on Wall Street thrives on a diet of fruit jam, bread, pasta, and coffee. She exercises intermittently. One day she decides to go to her primary healthcare provider for a routine checkup.
Calculating the Concentration of Acetic Acid in Vinegar Lab
A titration involves reacting two solutions - one with a known concentration and the other with an unknown concentration. The goal of the lab is to calculate the concentration of acetic acid in vinegar, so that is the solution with the unknown concentration. Therefore, there must be a way to find the concentration of the base
Determining Concentration of Mercury using Absorbance Values
Exercise: A sample of 1.500g of gold was analyzed to determine mercury in the same one. It was come by the method of standard addition preparing solution a standard Hg mercury (II) putting under it conditions similar to the one of the sample: Standard solution: An amount of 50 mg of pure mercury becomes to Hg (II) and is ad
Calorimetry and Specific Heat PreLab
Assume you use calorimetry to calculate the specific heat capacity of a 125.24 g piece of unknown metal. You intially heat the metal to 100.0 °C in boiling water. You then drop the chunk of metal into a calorimeter containing 47.22 g of water at 19.7 °C. After closing and stiring the calorimeter thoroughly, the metal and water
Spectrophotometry : Plotting Absorbance against analyte Concentration (Regression and Least-Squares Analysis)
The data in the table below were obtained during a colorimetric determination of glucose in blood serum. Glucose Absorbance, concentration, NM A 0.0 0.002 2.0 0.150
Lab Report : Alcohol Content of Vodka by Dichromate Titration
Here are the lab steps 1. Take a volumetric flask from the Glassware shelf and place it on the workbench. 2. Prepare a standard solution of potassium dichromate by adding 4g of dry potassium dichromate to the volumetric flask and filling the volumetric flask to the 100 mL mark with water. 3. Take a second volumetric fla
