Quantitative Analysis
BrainMass Categories within Quantitative Analysis
Beer Lambert Law
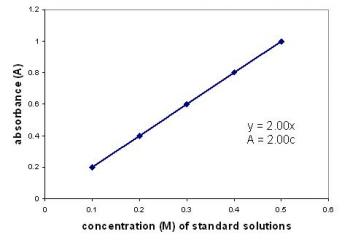
The Beer-Lambert Law describes the relationship between light absorption and the properties of the material which the light is travelling through.
BrainMass Solutions Available for Instant Download
Analytic Balance Lab
Need the answer to the following questions based on the data in the attached file : QUESTIONS: 1. From steps 1-5 in part C, compare the mass of the clean, dry bottle with the mass of the bottle after the sample has been removed. List possible reasons for any discrepancy. Suggest ways to test your hypotheses. 2. Explain step
Quantitative Analysis in Chemistry
I have attached a paper with two questions I am not sure how to approach. This is my first assignment in quantitative analysis. I would like to start off with a good understanding of the topic, so complete work and explanation would be greatly appreciated. Five mineral samples of equal mass of Calcite, 〖CaCO〗_3 (MM
Capillary Zone Electrophoresis, Molar Solubility and Titrations
1. Give an example of an absorption type stationary phase. 3. Explain why at pH around 7, the analyte anions move towards the cathode in capillary zone electrophoresis. 4. The Ksp of La(IO3)3 is 1.0×10-11. Using activities, what is the molar solubility of La(IO3)3 in a 0.050 M solution of NaNO3? 5. Calculate pFe2+ a
Quantitative Analysis - Equilibrium
1. In you book, the author states that K for carbonic acid is 4.5 à? 10 (see below) H2CO3(aq) ↔H+ (aq) + HCO3 (aq) K a1 = 4.5 à? 10-7 Is this a valid equilibrium constant for this reaction? Fully explain your answer. 4. Glycine, the simplest of the amino acids found in pr
Quantitative Analysis Calculations
Please solve each of the following problems with all calculations shown. 1. A 25.00 mL portion of a solution known to contain NaBr was treated with excess AgNO3 to precipitate 0.02578 g of AgBr. Determine the concentration of NaBr in the original solution in molarity and ppm. 2. How many mL of a 3.45% solution of alco
The Determination of Ascorbic Acid in Vitamin Tablets
1. This experiment involves a number of redox reactions. They are carried out in the following order of stages. To determine the amount of ascorbic acid in the tablet properly, the limiting reagent condition must be adhered to at each of the stages. Underline the reactant in each of the above reactions that is acting as t
