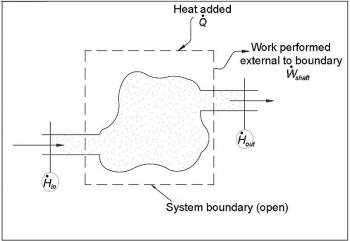
Thermodynamics is a branch of natural science concerned with heat and its relation to energy and work. It defines macroscopic variables, such as temperature, internal energy, entropy and pressure, which characterize materials and radiation. It explains how they are related and by what laws they change with time. Thermodynamics describes the average behavior of very large numbers of microscopic constituents. Its laws are derived from statistical mechanics.
Thermodynamics can be applied to a wide variety of topics in science and engineering such as engines, phase transitions, chemical reactions, transport phenomena and black holes. The results of thermodynamic calculations are vital for many fields including, chemistry, chemical engineering, aerospace engineering, mechanical engineering, cell biology, bio-medical engineering and material science.
Thermodynamics was developed out of the desire to increase the efficiency of early steam engines. In particularly, the French physicist Nicolas Leonard Sadi Carnot in 1824 who believed that the efficiency of heat engines was the key that could help France win the Napoleonic Wars. However, the Irish born British physicist Lord Kelvin was the first to formulate a concise definition of thermodynamics in 1854.
Initially, thermodynamics of heat engines were concerned mainly with the thermal properties of their working material, such as steam. This study was then linked to the study of energy transfers in chemical processes/ Chemical thermodynamics studies the role of entropy in chemical reactions. Statistical thermodynamics gave explanations of macroscopic thermodynamics by statistical predictions of the collective motion of particles based on the mechanics of their microscopic behavior.
© BrainMass Inc. brainmass.com June 30, 2024, 9:20 am ad1c9bdddf