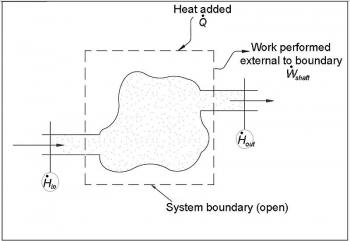
Internal energy is the total energy contained by a thermodynamic system. It is the energy required to create the system but remove the energy to displace the system’s surroundings, any energy connected with a move as a whole. The internal energy of a system can be changed by heating or cooling the system. The first law of thermodynamics states that an increase in internal energy is equal to the total heat added and work done by the surroundings.Therefore if a system is isolated, the internal energy cannot change.
Internal energy is a state function of a system because its value depends only on the current state of the system and not on the path taken or process underdone to arrive at the state. It is an extensive quantity. The SI unit of internal energy is a joule (J). Specific internal energy is the internal energy per unit of mass of the system in question.
Internal energy can be calculated by:
U= U_pot+ U_kin
And the change in internal energy is
∆U=Q+ W_mech+ W_extra
© BrainMass Inc. brainmass.com June 30, 2024, 9:26 am ad1c9bdddf