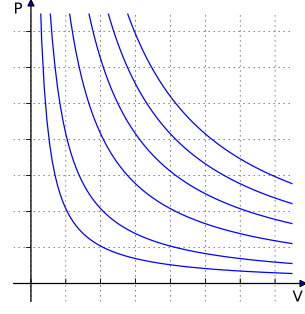
The kinetic theory of gases describes a gas as a large number of small particles which are in constant random motion. The particles constantly collide with each other and with the sides of the container. The kinetic theory explains properties of gases, such as temperature, pressure and volume. It considers the molecular composition and motion of the molecules. The theory states that pressure is not due to static repulsion between molecules but to collisions between molecules moving at different velocities through Brownian motion.
Gas particles are too small to be visible by the human eye. The jittering motions of pollen grains or dust particles which can be seen under a microscope are known as Brownian motion which display the same properties as gas particles. The results of this jittering motion is directly from collisions between the particles. This is experimental evidence for kinetic theory confirmed the existence of atoms and molecules.
© BrainMass Inc. brainmass.com June 28, 2024, 4:48 pm ad1c9bdddf