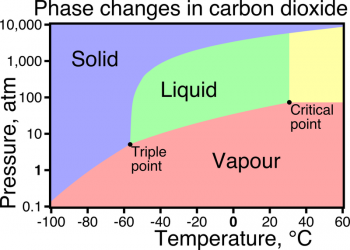
Specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius. The relationship between heat and temperature change can be seen below. However this relationship does not apply is a phase change occurs because the heat added or removed during a phase change does not change the temperature.
Q = cmΔT
Where Q is the heat added, c is the specific heat, m is mass, and ΔT is the change in temperature.
The specfic heat of water is 1 calorie/gram oC = 4.186 joule/gram oC. This is higher than any other common substance. As a result, water plays a very important role in temperature regulation. The specific heat per gram of water is higher than that for a metal.
The molar specific heat of most solids at room temperature and above is nearly constant. This comes from the Law of Dulong and Petit. At lower temperatures the specific heat drops as quantum processes become significant. The low temperature behavior is described by Einstein-Debye model of specific heat.
© BrainMass Inc. brainmass.com June 29, 2024, 12:18 pm ad1c9bdddf