Acids and Bases
BrainMass Categories within Acids and Bases
Acid Base Neutralization
An Acid-Base Neutralization is a reaction where an acid and base react to form a salt.
Ions and Salts
A salt forms when an acid reacts with a base.
BrainMass Solutions Available for Instant Download
Cleaning Pennies with Vinegar and Salt
In this task you will use the experimental scientific method to investigate a relevant, testable problem and communicate your findings in an organized written report. Design and carry out a scientific experiment that investigates a topic from either the life, earth or physical sciences and uses appropriate methods, tools, tec
Ranking Acidity and Hydrogen Atoms
The attachment contains all of the questions regarding ranking acidity and different forms of hydrogen atoms.
Calculating the pH of a weak acid
Given the concentration of a weak acid, calculate the pH of the solution. The Ka for acetic acid, CH3COOH, is 1.8x10^-5. Calculate the [H+] of a 0.20 M solution of acetic acid in water.
Understanding and Calculating Dilution Factors (DF)
Understanding dilution factors Question: A courier has just delivered a 2.0 mL sample of serum to your laboratory for analysis. In your analysis, you will combine a 0.05 mL aliquot of the serum with 9.95 mL of phosphate buffered saline diluent. Calculate the dilution factor.
Final pH of Tris
Calculate the final pH of 25 mL of buffer 0.1M Tris.HCl/Tris buffer, pH=6.60 after the addition of 1.00mL of 0.1M NaOH. pKa is 8.08. use the 0.10M Tris.HCl and 0.10M Tris to prepare 100mL, pH=6.6 solution. it calculated by pre-lab, it need 96.8 mL 0.10M Tris.HCl and 3.2mL Tris. and take 25mL pH=6.6 solution, then add 1mL of 0
Acid Reactions: Ion Concentrations and Ionization Constants
Find the solutions without using the Henderson-Hasselbalch equation. Please show all calculations. Question #1 A student dissolves 0.25 moles of the acid HA in water and dilutes the solution to the final volume of 500 mL. The student then gives a small portion of the solution to her professor. The professor uses a pH meter
Concentration without Hasselbach
Please find the solutions without using the Hasselbach equation. Please show all calculations. Question 1 Find [H^+] in a 25 degrees Celsius solution prepared by dissolving 0.20 moles of NH4Cl in water and diluting to a volume of 400 mL, given that Kb = 1.8 x 10^-5 for NH3 (aq) at 25 degrees Celsius. Question 2 Find
Air Bag Experiment
We did an experiment to determine how many grams of NaHCO3 and 0.8 M H2SO4 were required to inflate a quart-sized Ziploc bag. We determined the volume of the Ziploc bag to be 1.350 L. Using the ideal gas law, the molar calculation was 0.547 mols. We did 2 different scenarios. One was with the NaHCO3 as the limiting reagent, the
Reaction Mechanisms with resonance structures
1. Draw an arrow pushing mechanism for the reaction shown below. Be sure to show all lone pairs of electrons, all formal charges, all intermediates, and all important resonance structures 2. Provide reasonable synthetic sequences for the preparation of each of the following compounds from the indicated starting materials and
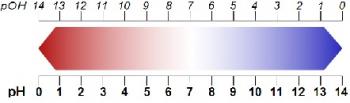
pH and pOH problems
a) If 0.1 g of Silver Nitrite (AgNO2; Ksp = 6.0 x 10 ^ -4) is added to 100 mL of water, what would the pH and pOH be? Note: The Ka for HNO2 is 7.2 x 10 ^ -4 b). If 0.1 g of Silver Nitrite is added to 0.1M solution of NaOH, what would the pH be? How much Silver Nitrite would dissolve?
Acid - Base Reactions Henderson-Hasselbalch Equation
Please show all work. Do NOT use the Henderson-Hasselbalch equation in solving any of these questions. 1. Ksp = 2 x 10^-19 for LaF3 in water at 25 degrees celcius. How many grams of LaF3 are present in 500 mL of saturated LaF3(aq) at 25 degrees celcius ? The atomic weight of La is 139. 2. Find [H+] in 0.25 M NaC2H3O2 (
General Properties of Aqueous Solutions
1- Do Strong electrolytes (in solution) conduct electricity well? 2- Determine if the following are soluble or insoluble in water PbCl2 PbNO3 PbS Na2S BaCO3 Ba(OH)2 LiOH AgCl AgC2H3O2 AgOH 3-Write the balanced net ionic equation for the following reaction: Na2SO4 + Pb(NO3)2--> 4-Write the balanced net io
Find pH and Mass Salt Mixture
1. Carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved CO_2. Dissolved CO_2 satisfies the equilibrium equation The acid dissociation constants listed in most standard reference texts for carbonic acid actually apply to dissolved CO_2. For a CO_2 partial pressure of 7.7×10-4 atm in the a
Examples of Strong and Weak Acids and Bases and Buffers
This solution is an answer to finding the pH of a solution if it is either a strong acid/base or a weak acid/base or a buffer containing a weak acid and its conjugate or weak base and it's conjugate. 1. a) Give examples of strong acids and bases. b) find the pH of 0.01 M and 0.001 M solution of each. 2. a) Give exa
Electrochemistry: Oxidation Reactions
Please explain how to do these E-chem reactions. Set 1: Find the standard reduction potential for the reaction (it's a range based off results) VO3-(aq) + 4H+(aq) + e- → VO2+(aq) + 2H2O(l) Ecell=?? Results for my reactions any heat that is mentioned was for 12-15 minutes Vanadyl Sulfate (blue solution): 1.
Finding K
I have some problems on solving for K in equilbrium. I need a clear understanding for a test coming up early next week. I would appreciate a complete explanation with the work. Thank you for the help. 1. At 22 °C an excess amount of a generic metal hydroxide M(OH)2, is mixed with pure water. The resulting equilibrium solut
General Chemistry: Various Elements and Metals
Hi, I need some assistance with the following chemistry questions. I am really struggling with these different concepts. 1. Bromine is a reddish-brown liquid with a relatively low boiling point. What is the density (g/mL) of bromine if 861 mL has a mass of 2686 g? 2. How many liters of bromine are present in 85 mL of bro
The Behavior of Substances in Water
4.19. The Behavior of Substances in Water Part 1 a. Ammonia, NH3, is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this process, including state symbols. b. From everyday experience you are probably aware th
How to Prepare an HCl Solution
Prepare a step by step process to prepare 500 grams of a 5% HCl aqueous solution. (Assume 37% by mass HCl stock solution is used)
pH Observations: Example Problem
You measure the pH (which is a measure of the [H+], the lower the pH the higher the [H+] is) of a pure water solution and find that it has a pH of 7.0; however, after sitting for a while on the desk, the pH is taken again and found to be 5.7. If this solution is then heated, cooled to room temperature again, the pH is again 7.0.
Chemistry Problems: Equilibrium Concentration of Gas
A 3.00 L flask initially contains 1.50 mol of gas A and 0.450 mol of gas B. Gas A decomposes according to: 3A <-> 2B + C DeltaH = +90kJ mol^-1 The equilibrium concentration of gas C is 0.100 mol/L. What is the equilibrium concentration of gas A? What is the equilibrium concentration of gas B? What is the equilibrium co
Herbicides and the Effects on Plants
Assignment CROP 440/540 Week 7 A field corn grower near Mythtown, Central State, has called, and asked you to visit a field that he is worried about. He says the entire field looks sick, and he wants you to give an expert opinion on what you think is wrong. During your conversation, you learn that about a week ago, the grower a
Products Produced by the Oxidation of C7H14O3
How many grams of the products are produced by the oxidation of C7H14O3 by potassium permanganate in acidic solution (HCl is the acid) when you start with 25.65494 g of C7H14O2 and stoichiometric amounts of potassium permanganate? The products are CO2, H2O, the manganese(II) ion, and of course the potassium and chloride ions, wh
Oxyacids: Oxygen and Strength of the Acid
Several elements form oxyacids with different numbers of oxygens attached to the central atom. However, the strongest of these oxyacids are the ones that have the most oxygens attached. For example, HNO2 is a weak acid; whereas, HNO3 is a very strong acid. Likewise, H2SO3 is a weak acid and H2SO4 is a very strong acid. Using
Resolution of Mixtures: Filtration and Distillation
The experiment performed is outlined in the attached pdf file. I did not do the melting point or silver nitrate parts, but I would still like to know what was going on if I did. Please help me with citations as needed, so I can look them up for more detail. Purpose: The separation of mixtures into their constituent components
Hydronium ion
The authors of your book use the hydronium ion to indicate what happens when an acid has been dissolved in water. Is this really a legitimate representation of what happens when an acid dissolves in water? Fully explain your answer.
Chemistry - Determining Error
Sodium bicarbonate was added to an unlimited amount of sulfuric acid undergoing a reaction to produce sodium sulfate plus carbonic acid (which turned in to water vapor and carbon dioxide upon heating). Na2CO3 +H2SO4------> H2CO3 +Na2SO4 I would like to know: a.) 2.095 grams of sodium bicarbonate was used in the reaction an
Empirical formula of a compound consisting of lead, carbon, hydrogen and oxygen
An organic compound containing lead was found to contain 21.70 C and 2.7% H. The lead content was found by dissolving 4.6201g of the compound in water and adding sodium sulfate. 3.100 g of lead precipitate was collected. What is the empirical formula of the compound? [assume the compound consists of lead, carbon, hydrogen and
Molar Masses
BACKGROUND Here is an example of how to make up a W/V solution, to give you an idea about the steps for the next exercise. Using the salt provided to you, you can make a 50 ml of 5% (W/V) NaCl solution. *Steps: 1. Get a clean 250ml-beaker ready; 2. Add about 35 ml dH2O to above beaker; 3. Weigh _______ grams of salt usin
Chemicals Left in Water after Firefighters Leave
Why do firefighters recommend the use of a deluging volume of water when extinguishing an oxidizer-supported fire? What may happen to a large (i.e., 30-50lb) container of trichloro-s-triazinetrione, or calcium hypochlorite, if it is left to sit in the water and muck remaining in a building after fire fighting efforts have ended,
