Chemical Equilibrium
BrainMass Categories within Chemical Equilibrium
Le Chatelier’s Principle
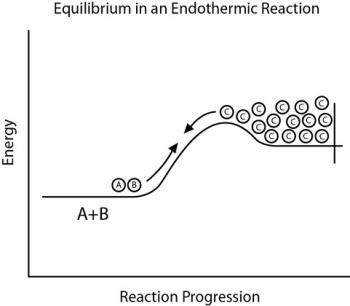
Le Chatelier’s Principle can be used to predict how a change in condition affects a chemical equilibrium.
BrainMass Solutions Available for Instant Download
Blood and hemoglobin
Essay Topic: (Virtual Lab report) Life at high altitudes and haemoglobin production. More details about tasks is attached in document below: Length: 750 words (calculations are not included in the word limit) Topic: Virtual Lab Report requirements: - A minimum of 5 references. These can include journals, books, reput
Gibb's Phase Rule
I have a problem understanding the degrees of freedom for two binary diagrams because they have different degrees of freedoms in some parts of the diagram and I don't get why they have that. It would be great if you could explain if this is because of less chemical potential equilibrium or from less variables from the derivation
Equilibrium Constant Expression
Given the following reaction: C0(g) + H2O(g) <> C02(g) + H2(g) (Please see the attachment for the properly formatted reaction). a) Write the equilibrium constant expression for this reaction. Note: gaseous-phase H20 is included in the equilibrium expressions, unlike liquid phase H20. b) The equilibrium constant for the reactio
Cell Reactions & Gases
Question #1 The gas reaction N2(g) + 3H2(g) <--> 2NH3 (g) has K- = 0.80 at 600 K and Kp = 0.15 at 650 K a) Is delta H greater than or less than zero? b) Is the reaction exothermic or endothermic? Question #2 The reaction N2 (g) + 3H2 (g) <---> 2HN3 (g) is at equlibrium a) The number of moles N2 present plus he numb
Equilibrium Constant of SO2 and SO3
The standard free energies of formation of SO2(g) and SO3(g) are -300.4 and -370.4kJ mol-1, respectively. Calculate the value of the equilibrium constant at 25'C for the reaction 2SO2(g) + O2(g) <=======> 2SO3(g)
Equilibrium Concentrations in a System
For the chemical system shown below, it was found that [N2] = [O2] = 0.70 mol/L and [NO] = 0.22 mol/L, at equilibrium. Suppose that enough N2 was added to increase its concentration temporarily to 1.00 mol/L. When the system reaches equilibrium, what will be the new equilibrium concentrations of N2, O2, and NO? N2(g) + O2(g) ?
Boiling Point Determinations for Chemistry Students
1) Why is a mixture of ice and water, rather than ice alone, used in calibrating a thermometer? 2) Why does the boiling point of a liquid vary with the barometric pressure? 3) What does it mean to say that the normal boiling point of a pure liquid substance is a characteristic property of the liquid substance?
Envirnomental Gases
I have attached a problem I am not really sure how to approach. I would appreciate a complete explanation and work. ** Please see the attached file for the complete problem description **
Chemistry: Pb(NO3) mixed with M in NaBr
Will 0.060 M in Pb(NO3) mixed with 0.020 M in NaBr form a precipitate? (Explain)
Determining the equilibrium constant for a reaction
For the reaction: Kc = 255 at 1000k CO (g) +Cl2 (g) == COCI2 (g) If a reaction mixture initially contains a CO concentrations of 0.1500 M and a Cl2 concentration of 0.175 M at 1000 k, what are the equllibrium concentrations of CO, Cl2, and COCl2 at 1000 K?
General Chemistry Multiple Choice Questions with Answers and Explanations: Molarity, Acids, Bases, Equilibrium.
The minimum combined kinetic energy reactant particles must possess in order for their collision to result in a reaction is called the Answer A. collision energy. B. orientation energy. C. activation energy. D. dissociation energy. Question 2 The defini
Solevent extraction solvent system
1. What are the advantages and disadvantages of using ether as an extraction solvent? 2. Why must the stopper at the top of the separatory funnel be removed before liquid can be withdrawn through the stopcock? The detailed description of the above mentioned problem is described in the attached file.
Equilibrium Reaction of Insoluble Water
Lead(II) carbonate is very insoluble in water, meaning that the equilibrium constant for the dissociation reaction is much less than 1: PbCO_3 (s) <-> Pb(2+) (aq) + CO_3(2-) (aq) Write reactions for each of the following and explain how the equilibrium reaction for the dissociation of lead(II) carbonate is affected. a.
Equilibrium Constant Expressions
What are the equilibrium constant expressions for the following? a) PCl 5 (g) <=> PCl 3 (g) + CI 2 (g) b) Cu (OH) 2 (s) <=> Cu 2+(aq) + 20H- (aq) c) [Cu (NH3)4]2+(aq) <=> Cu 2+(aq) + 4NH3 (aq) d) CH3 CH2 CH2COOH (aq) + H2O (l) <=> CH3 CH2 CH2COO- (aq) + H3O+(aq)
Distribution Coefficient Question
When an aqueous solution of an organic compound is shaken with an immiscible organic solvent, such as diethyl ether, the solute distributes itself between the two phases. When the two phases separate into two distinct layers, an equilibrium will have been established such that the ratio of the concentrations of the solute in eac
Reaction system: Concentration and equilibrium
For the equilibrium: PCl5(g) PCl3(g) + Cl2(g) Kc = 4.0 at 228°C. Pure PCl5 is added to the reaction system. At equilibrium there is 0.16 M PCl5. What is the concentration of PCl3 in this system? Answer = .80M But what are the steps to get this answer?
Ketones and Aldol Condensation
Ketones also undergo the aldol condensation although a successful reaction often requires "enhanced" conditions, as the addition involves an unfavorable equilibrium constant. This is the situation in the reaction in which 4-nitrochalcone is synthesized. With the odds against it, why is the reaction successful in this case?
Chemistry II: Chemical equilibrium Constant expressions
I need help with the following problem with steps included: Consider the following reaction: N2(g) + O2(g)ï? 2NO(g) If the equilibrium partial pressures of N2, O2, and NO are 0.15 atm, 0.33 atm, abd 0.50 atm, respectively, at 2200oC what is Kp? I have also attached the problem if you need to see the correct form
Reaction that generates 4-hydroxycoumarol
Water must be excluded from the reaction that generates 4-hydroxycoumarol. In the first step of this reaciton, formation of 4-hydroxycoumarin, what is the purpose of removing the ethanol generated in the reaction?
A stoppered flask at 25 C contains 250 mL water, 200 mL octanol, and 50 mL of air.
A stoppered flask at 25 C contains 250 mL water, 200 mL octanol, and 50 mL of air. An unknown mass of o-xylene is added to the flask and allowed to partition among the phases. After equilibrium has been established, 5.0 mg of o-xylene are measured in the water. What is the total mass (g) of o-xylene present in the flask?
Equilibrium constant (Kc) for the reaction
At 1130oC the equilibrium constant (Kc)for the reaction: 2H2S(g) <----->2H2(g) + S2(g) Is 2.25 X10-4, if [H2S]=4.84 X10-3 M and [H2]=1.50X10-3 M, calculate [S2]. ( need help with the following problem with the steps)
Hydrolysis and C=O sub.
Questions: 1.) Show what compounds result from hydrolysis of the following compound. 2.) Draw the major product of the following reaction (after aqueous workup). Please see the attachment for the visual components. Thanks for all of your help!
equilibrium constant Ke
Find the value of Ke for the reaction, A(g) + 2B (g) ---> 2C(g) + D(g) In which it was found that at equilibrium, [A] = 0.200 mol/L, [B] = 0.200 mol/L, [C] = 0.0500 mol/L, and [D] = 0.0750 mol/L.
Kb for Methylamine and Chemical Equations
The Kb for methylamine, CH3NH2, is 4.4 x 10-4 at 25°C a) write a chemical equation for the equilibrium that corresponds to Kb b) by using the value of Kb, calculate ΔG° for the equilibrium in part (a) c) what is the value ΔG at equilibrium ? d) What is the value of ΔGwhen [H+] = 1.5 x 10-8 M, [CH3NH3+] = 5.5 x 10-4 M, a
Calculate an equilibrium constant using percent dissociation
Can you show me how to do this one? Thank you at 1600°C. Br2(g) = 2 Br(g) When 1.05 moles of Br2 are put in a 0.980 L flask, 1.20 percent of the Br2 undergoes dissociation. Calculate the equilibrium constant Kc for the reaction.
Solve for Equilibrium Constant
Do I just plug in the numbers to get the answer? Do I convert the temp to Kelvin? N2(g) + O2(g) = 2 NO(g) If the equilibrium partial pressures of N2, O2, and NO are 0.15 atm, 0.33 atm, and 0.050 atm, respectively, at 2200°C, what is KP?
Equilibrium Reactions of Mixtures
Just when I thought I was catching on.... then I missed this question by more than a factor of 10.....Help! For the reaction given below at 700°C, Kc = 0.534. H2(g) + CO2(g) = H2O(g) + CO(g) Calculate the number of moles of H2 that are present at equilibrium if a mixture of 0.297 moles of CO and 0.297 moles of H2O is
Kp and Kc equations
2CO(g)+O2(g)=2CO2(g) If Kc is 2.24 e22 at 1273 degrees C what is Kp I know it must seem silly, but I still can't figure out how to set it up. Can you show me again?
Reverse Reaction Equilibrium
The equilibrium constant (Kc) for the reaction shown below is 4.17x 10-34 at 25°C. 2 HCl(g) = H2(g) + Cl2(g) What is the equilibrium constant for the following reaction at the same temperature? H2(g) + Cl2(g) = 2 HCl(g) Why isn't it the same Kc? Is it the inverse?
Equilibrium constant Kc
This is one of the ones that I didn't get right. Can you show me how to do it? H2(g) + Br2(g) = 2 HBr(g) Begining with 2.50 moles at 730 degrees C in a 12.o liter vessel, if the Kc is 2.18 e6 ............what are the concretration of H2, Br2 and HBr at equilibrium
