Energetics and Thermodynamics
BrainMass Categories within Energetics and Thermodynamics
Phase, State and Energy Changes
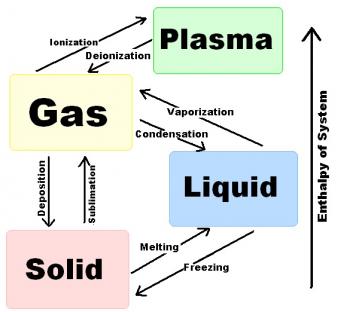
Phase and State Changes refer to the transformation of a thermodynamic system from one phase or state to another.
Reaction Intermediates and Mechanisms
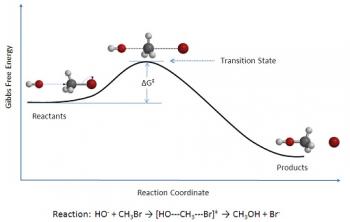
In a Reaction Mechanism, a Reaction Intermediate is a molecular entity that is formed by the reactants which reacts further to form the products.
Hess’ Law
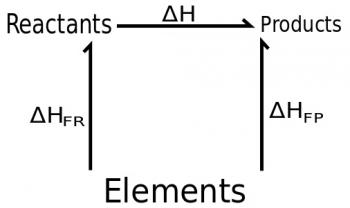
Hess’s Law states that the total enthalpy change accompanying a particular chemical reaction is independent of the pathway between the initial and final states.
Raoult's Law
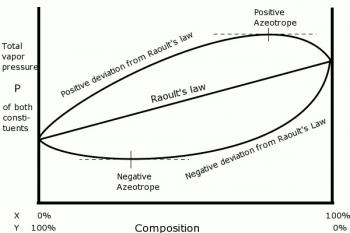
Raoult’s Law states that the vapor pressure of an ideal solution is dependent on the vapor pressure and mole fraction of each chemical component in the solution.
Law of Conservation of Mass
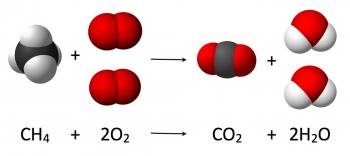
The Law of Conservation of Mass states that for any system closed to all transfers of mass and energy, the mass of the system must remain constant over time.
BrainMass Solutions Available for Instant Download
Entropy change at constant pressure and volume
1) Find the standard enthalpy of cyanamide (CH3N2 (s) ) from its heat of combustion to CO2 and H2O of -741.40kJ/mol. 2) Calculate delta S if the temperature of 2.50mol of an ideal gas with Cv = 5/2R is increased from 160K to 675k under conditions of a. constant pressure b. constant volume
Entropy of an ideal gas
1) One mole of an ideal gas is changed from 273.15K and 2 atm to 233.15K and 0.4 atm. Calculate delta S for this change in state. 2) Arrange the following chemicals in order of increasing entropy at STP. n - pentane (g) methanol ( l) ethanol (l) ethanol (g)
Isothermal and Adiabatic Expansion
One mole of an ideal, monoatomic gas undergoes the following processes: - Reversible, isothermal expansion from 10 atm to 2L and 5 atm ; - Adiabatic expansion from 10 atm to 2L and 5 atm ; Calculate q , w , change in U, and change in H for each process.
Expansion of gas
Question: The volume of 1 mole of an ideal, monoatomic gas which is initially at 2 atm, 25"C, and 12.2L is doubled by the following paths: a) isothermal expansion; b) adiabatic expansion; c) expansion along a path defined by P=0. 2V+b, where p is in atm, Vis in L/mol, and b is a constant. Assume that all paths are
Sublimation of CO2
The sublimation temperature of solid CO2 is -78.5 "C at 1 atm pressure. At what minimum pressure must gaseous CO2 be stored in a fire extinguisher at 25 degrees C to ensure that some "snow" is produced when the gas is released to atmospheric pressure? Use mico JT = 1.3 for CO2 and assume that it is independent of pressure and
Calculating Volume in Reversible Isothermal Expansion
An ideal gas undergoes a reversible isothermal expansion from an initial volume V1 to a final volume that is ten times greater, and in this process it does 10kJ of work, The initial pressure was 10^6 Nm^-2. a) Find V1 b) If there were initially 2 moles of the gas, what was its initial temperature?
Vapor Pressure of Mixtures of Miscible vs. Immiscible Liquids
Consider a physical explanation for the difference of vapour pressure of miscible and immiscible solutions. For immiscible the total vapour pressure is equal to the sum of the pressure from the pure liquids but and miscible liquids pressure vapour pressure follows raoults law. What is the physical justification for this?
Mathematically defining that a process is reversible
I have a question about relations for a process even though I am unsure about the scope of this. Still I wonder about a mathematical relation for the following: I wonder about how one defines mathematically that a process is reversible for a process that has both pressure and temperature differences from definition of total e
Gas Constant and Wavelength
Question #1 Given the following data from a student, who completed the Gas Law Experiment calculate the gas constant, R Mass of magnesium = .026g Initial syringe volume = 0.0 mL Final syringe volume = 23.5 mL Temperature = 23.0 degrees Celsius Barometric pressure = 764.2 mm Hg Question #2 Calculate the wavelength
Specific Heat & Enthalpy
Question #1 A 25.000g sample of unknown metal is heated to 99.5 degrees Celsius and added to 50.0mL of water in a calorimeter, which has an initial temperature of 22.o degrees Celsius (density of water is 0.99780 g/mL. The temperature of the calorimeter increases to a maximum temperature of 33.5 degrees Celsius. The heat capa
A Discussion On Delta S Values
Using the data sheet (see attachment) calculate delta S values for the following reactions. In each case explain the sign of delta S. N2H4(g) + H2(g) --> 2NH3(g) 2Al(s) + 3Cl2(g) --> 2AlCl3(g) Mg[OH]2(s) + 2Hcl(g) --> MgCl2(s) + 2H20(l) 2CH4(g) --> C2H6 + H2(g)
Gas Laws & Heat Of Reaction
1. For the reaction 2 NO(g) + O2(g) -->2 NO2(g) deltaH = -126.0 kJ at 25C. (a) Calculate the amount of heat transferred when 333 g of NO(g) reacts with O2(g) at 25C and 1 atm. (b) Does this heat flow into the system or does it flow to the surroundings? (c) Is this reaction exothermic or endothermic? 2. If 8675 J of
Two problems that involve calculating the value of a freezing point and to prove a statement about A statistical thermodynamic model of binary solutions.
1. If the vapor pressures of the two components in a binary solution are grven by P1 = x1*P1*e^(u(x2^2))/RT and P2 = x2*P2*e^(u(x1^2))/RT Show that, Del(mix)*G/u = Del(mix)*G/(n1+n2)u = (RT/u) * (x1 ln(x1) * x2ln(x2))*x1x2 Del(mix)*S/R = Del(mix)*S/(n1+n2)R= -(x1 ln(x1) * x2ln(x2)) Del(mix)*H/u = Del(mix)*H/(n1+n2) = x1
Van der Waals Gas Equation
The equation of state of one mole of a van der Waals gas is given by (P+a/(v^2))(V-b) = RT with a and b are constants. a) Calculate the work W in an isothermal reversible process when volume changes from V1 to V2. b) Using the energy equation, show that (du/dV) = a/v^2 c) Calculate the change in internal energy U in th
Molar Mass of Butane
1-HgO(s) Hg(l) + O2(g) Consider the unbalanced equation above. A sample of impure mercury(II) oxide is heated and the HgO decomposed completely. If 680. mL of O2 is collected by displacement of water at a barometric pressure of 680.0 mm Hg and 25.0°C, what mass of HgO was originally present? The vapor pressure of water is 23.
Enthalpy and ratio of slopes and graph
1. Consider the phase change: C(graphite) <---> C(diamond) Given that delta_rG^o/Jmol^-1 = 1895 + 3.363T, calculate the enthalpy and entropy. Calculate the pressure at which diamond and graphite are in equilibrium with each other at 25 degree Celsius. Take the density of diamond and graphite to be 3.51 g/cm^3 and 2.25 g/c
Gibbs Free Energy For Combustion of Octane
Calculate delta G combustion at 25'C of octane, c8H18(l), 2C8H18(l) + 25 O2(g) -------> 16 CO2(g) + 18 H2O(l) Using the following thermodynamic data and that given in textbook. For C8H18(l), delta H formation = - 208.4 kJ mol-1 and delta S = 463.71 JK-1 mol-1. Give your answer in kJ mol-1 of C8H18 (l).
Refrigerators: Liquid Ammonia and Heat Energy
Liquid ammonia, NH3(l), was once used in home refrigerators as the heat transfer fluid. The specific heat of the liquid is 4.7 J/g.K and that of the vapor is 2.2 J/g.K. The enthalpy of vaporization is 23.33 kJ/mol at the boiling point. If you heat 12 kg of liquid ammonia from -50.0 °C to its boiling point of -33.3 °C, allow i
Determining Boiling Points at Different Pressures
Hello, How do I determine the change in boiling point of methyl ethyl ketone (MEK) when initially at atmospheric pressure ---- therefore B.P. - 79.6 oC ------ and then under -27 inches of vacuum pressure? What would be the new B.P. of the MEK? Please please explain how to do this.
Thermal Interactions and Enthalpy
Thermal Interactions Part 1: In an insulated container, you mix 200. g of water at 80ºC with 100. g of water at 20ºC. After mixing, the temperature of the water is 60ºC. a. How much did the temperature of the hot water change? How much did the temperature of the cold water change? Compare the magnitudes (positive values) o
Enthalpy Change for Combustion
Estimate the enthalpy change for the combustion of one mole of ethanol (C2H5OH) using average bond dissociation enthalpies (in KJ/mol) for C-H: 413, O-H: 463, C-O: 358, C-C: 154, C=O: 122, and O=O: 498. Then use the estimate to calculate the heat of formation of ethanol from the heats of formation (in KJ/mol) of CO2: 394, and H2
Octahedral Metal Geometry
1. Some octahedral metal complexes are known to undergo the dissociative mechanism shown below. Consider both a d8 strong field complex and a d8 weak field complex. Would either of these complexes be expected to undergo this type of mechanism? Explain your reasoning. Assume P < 0.8d(delta)o for the strong field case. 2. List
Solving Enthalpy Changes for Chemical Reactions
Calculate the enthalpy change for the following reactions P4O6 + 2O2 ? P4O10 given the following enthalpies of reaction. P4 + 3O2 ? P4O6 ?H = -1640.1 kJ equation 1 P4 + 5O2 ? P4O10 ?H = -2940.1 kJ equation 2 b) NO2(g) + (7/2) H2(g) ---> 2H2O(l) + NH3(g) 2NH3(g) ---> N2(g) + 3H2(g) ?H
Heating a pot of water with pentane
You are out camping with your family and you decide that you would heat some water for cooking your food. The pot you use is made from aluminum (heat capacity, s, = 0.902 J/g@EC). The pot has a mass of 675.6 g and a capacity of 1.356 L. You are going to heat water (s = 4.184 J/g degrees C) starting from ice (s = 36.93 J/mol degr
Spontaneous and Non-Spontaneous Reactions
Chemical Species DeltaHf(kJmol^-1) DeltaGf(kJmol^-1) DeltaS(Jmol^-1K^-1) SO2(g) -296.8 -300.1 248.2 Cl2(g) 0 - 223.0 SO2Cl2(g) -364.0
Enthalpy Calculations for Solid Ammonium Nitrate
When a 3.88 g sample of solid ammonium nitrate dissolves in 60.0 g of water in a coffee-cup calorimeter, the temperature drops from 23.0 degrees Celsius to 18.4 degrees Celsius. Calculate delta H in kJ per mole of NH4NO3 for the solution process. Assume the specific heat of the solution is the same as that of pure water. Ple
Heating a pot of water with butane gas
You want to heat a pot of water. If you assume that the pot is made out of iron, which has a mass of 11.00 kg with a specific heat capacity (s) of 0.4498 J/g C, and you have exactly 1 L of pure water, starting with the water at exactly 15C and wanting to get the water to boil, how many grams of butane gas (C4H10) will need to b
Work Efficiency for a System
You have a system with a movable piston that is isolated. The reaction inside the system is under adiabatic conditions. When the reaction (the burning of butane in oxygen) is started, the distance the piston traverses is 30.00 cm at 1.000 atm of pressure, the diameter of the piston is 8.000 cm. If you take 15.00 mg of butane an
Kinetic Energy and Molecules
You have a sample of a substance at a given temperature and the temperature of a substance is proportional to the kinetic energy of the molecules. However, do all the molecules have the same energy? Fully explain. (Answer provided in less than 100 words.)
Is ethanol or gasoline more cost efficient?
As all of you are aware, there is a big push to use ethanol (C2H6O) as a source of fuel for our automobiles, instead of gasoline. Assuming that gasoline can be adequately represented by octane (C8H10), determine how reasonable it is. That is, will individuals actually save any money using ethanol (at approximately $0.83/L) as
