In a Reaction Mechanism, a Reaction Intermediate is a molecular entity that is formed by the reactants which reacts further to form the products. Most chemical reactions require more than one step to form the products; and thus a reaction intermediate is almost always formed during the course of a reaction. However, these intermediates are often short-lived and are very difficult to isolate, as they are frequently very reactive.
For example consider the reaction:
A + B --> C + D
The above reaction may hypothetically proceed as follows:
A + B --> X1 + X2
X1 + X2 --> X3
X3 --> C + D
The species X1, X2 and X3would all be considered reaction intermediates, as they are neither the reactants nor the products, but are formed during the course of the reaction.
On an energy diagram a reaction intermediate can be identified by a valley in between the reactant and products (Figure 1).
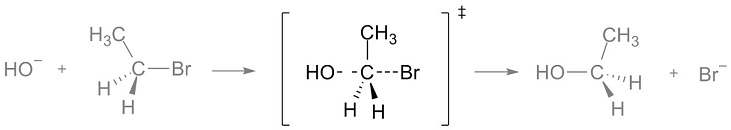
Figure 1. Reaction Intermediate
Title Image Credit: Wikimedia Commons
© BrainMass Inc. brainmass.com June 30, 2024, 10:13 am ad1c9bdddf