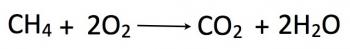
Stoichiometry refers to the study of relative quantities of reactants and/or products within a defined chemical system. It focuses on the relationships among quantities of reactants and products usually forming ratios of positive integers due to the nature of the bonded atoms. For example, in the combustion of methane (CH4), exactly 1 mole of methane reacts with 2 moles of oxygen to form 1 mole of carbon dioxide and 2 moles of water:
CH4(g) + 2O2(g) --> CO2(g) + 2H2O(l)
Considering the relative abundances of reactants and products is a specific branch of stoichiometry called ‘reaction stoichiometry.’ In the example above, reaction stoichiometry describes the 1:2:1:2 ratio of molecules CH4, O2, CO2 and H2O. These ratios can be used to determine values such as the percentage yield of products under different conditions, the presence of a limiting reagent, and even predict the behavior of certain elements under experimental conditions.
Thus, understanding the fundamentals of stoichiometry can help calculate the desired quantitative data regarding chemical reactions, as well as other value which do not pertain to chemical reactions, such as the examination of elements within compounds and the behavior of gases.
© BrainMass Inc. brainmass.com June 30, 2024, 12:10 am ad1c9bdddf