Oxidation-Reduction and Electrochemistry
BrainMass Categories within Oxidation-Reduction and Electrochemistry
Redox Reactions
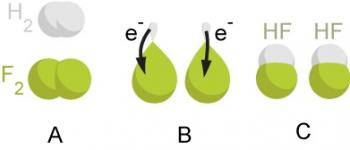
Redox Reactions refer to a set of chemical reactions which consist of an electron transfer and a change in oxidation state of the reactant species.
Electrochemical Cells
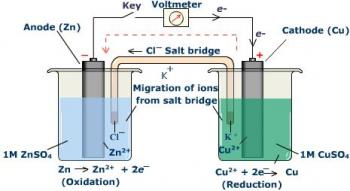
An Electrochemical Cell is an experimental system capable of either generative electrical energy from two half cells, or facilitating the chemical reaction between two half cells through the introduction of electrical energy.
Electrochemical Potentials
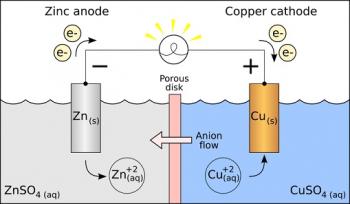
The Electrochemical Potential is a measurement of the energy stored in the chemical potential of redox reactions.
Batteries
A Battery in electrochemistry refers to an electrochemical cell with the capability of producing and storing electrical energy.
Corrosion

Corrosion, in electrochemistry, is the gradual destruction of an electrode caused by an electrochemical process.
Electrophoresis
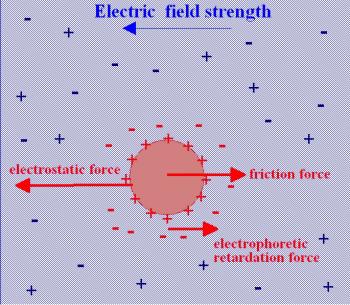
Electrophoresis refers to the movement of dispersed charged particles under the influence of an applied uniform electric field.
BrainMass Solutions Available for Instant Download
Oxidation or Reduction of Lead
When lead is converted from its metallic form to an ionic form, are electrons lost or gained? Is this oxidation or reduction? I believe it is oxidation but how do I show it in equation form? Pb + PbO2 + 4 H+ + 2 SO42- double arrow 2 PbSO4 + 2 H2O.
Strong/Weak Field Isomers
Question 1 [COF6]^3- is a weak-field ion, and [Co(CN)6]^3- is a strong-field ion. Which ion absorbs higher-frequency visible light? a) [COF6}^3- b) [Co(CN)6]^3- Question #2 Give the formula of potassium diaquatetrachlorocobaltate (II). Question # 3 Draw all isomers with the formula [Co(H2O)4Cl2]
Molybdenum enzyme model compounds .
(a) The following complexes I, II, and III represent model compounds for the molybdenum-containing enzymes involved in oxygen-atom transfer (OAT) reactions. In each case assign the formal oxidation state of the metal centre and clearly show your reasoning: **Please see the attached document for the diagrams.** (b) So
Ferredoxins and Redox Couples
a) Comment on the applicability of [Fe2(S)2(SPh)4]^n shown below as a model for redox/spin states of [2Fe*2S] ferredoxin, given that the observed values of the effective magnetic moments are 0 and 1.75 for [Fe2(S)2(SPh)4]^-2 and [Fe2(S)2(SPh)4]^-3respectively b) Explaining your reasons, use the ^57Fe Mossbauer data below to
Glucose and Vanadium Pentoxide
With how many grams of each reactant (glucose and vanadium pentoxide) did you start and with how many grams of each product were produced for the oxidation of glucose (C6H12O6) by vanadium pentoxide in an acidic solution. The products are CO2, V3+, and H2O. You place stoichiometric amounts of the reactants into an evacuated 2.0
Energy from Hydrogen
Calculate the minimum energy required to produce molecular hydrogen from gas-phase water. Then compare this to the energy need to produce water electrochemically or photoelectrochemically. Finally, compare this to the energy needed to make this on a zirconia surface. Comment on why there are differences.
Redox Titration-Analysis of Bleach using Potassium Iodide
To determine concentration of sodium thiosulfate solution as in this experiment, a student pipetted 25.0 mL of 0.0100 M potassium iodate (KIO3) solution into a 125 mL Erlenmeyer flask. Potassium iodide and sulfuric acid were added as in this experiment. The titration needed 15.21 mL of the sodium thiosulfate to reach the end poi
Redox Titration-Analysis of Bleach (Sodium Hypochlorite)
The active ingredient in many commercial liquid bleaches is sodium hypochlorite. The bottle lists the percentage of sodium hypochlorite as 6.0%. If the density of commercial bleach is 1.084 g/mL, how many mL of 0.150 M sodium thiosulfate is required to reach the end point in a titration similar to the one performed in this exper
Alcohol consumption word problem
A 40-year-old manager, at a prominent legal firm in L.A., experiences abdominal pain post lunch. He goes to the restroom a couple of times, but continues to experience pain and cramps. He eventually decides to get himself examined. While questioning him, you learn that he consumed two bottles of beer at lunch. The alcohol (ethyl
Balancing Half-reactions Red-Ox
Use the following pair of half-reactions to design a galvanic cell. Then write in the proper coefficient for each of the species involved in the overall reaction. Water molecules and protons are not shown in the half-reactions, but they may be needed to the overall reaction. N2(g)->N2H5+(aq) E=-.023V MnO4-(aq) -> Mn2+ (
Balanced Molecular Ionic Net Equations
1. Calculate the concentration of a Pb(NO3)2 in a solution made up of 49.1 mg of solid Pb(NO3)2 (MW=33.22) dissolved in 65.00 mL of water (MW=18.02). 2. Write balanced molecular, ionic, and net equations for the following reactions. a. KCN(aq) + MgCI2(aq) - KCL (aq) +Mg(CN)2(aq) b. Zn(NO3)2 (aq) +H3PO4(aq)
I need to solve this problem
A 40-year-old manager, at a prominent legal firm in L.A., experiences abdominal pain post lunch. He goes to the restroom a couple of times, but continues to experience pain and cramps. He eventually decides to get himself examined. While questioning him, you learn that he consumed two bottles of beer at lunch. The alcohol (ethyl
Reducing and Oxidizing Substance Equations
14. Tell which substance is reduced and which is oxidized in the following equations. Label the oxidizing agent and reducing agent in each equation. a. 2Al(s)+3Cl_(2 ) (g) ï? 2AlCl_3(s) b. S(s)+O_2(g)ï? SO_2(g) c. CuO(s)+H_2(g)ï? Cu(s)+H_2O(g) d. C_2 H_4(g)+H_2(g)ï? C_2 H_6(g)
Need Help on this one.
14. Tell which substance is reduced and which is oxidized in the following equations. Label the oxidizing agent and reducing agent in each equation. a. 2Al(s)+3Cl_(2 ) (g) ï? 2AlCl_3(s) b. S(s)+O_2(g)ï? SO_2(g) c. CuO(s)+H_2(g)ï? Cu(s)+H_2O(g) d. C_2 H_4(g)+H_2(g)ï? C_2 H_6(g)
Oxidized and Reduced Species
Which element is oxidized and which is reduced in the following reactions? N2(g)+3H2(g)>>2NH3(g) 3Fe(NO3)2(aq)+2Al(s)>>3Fe(s)+2Al(NO3)3(aq) Cl2(aq)+2NaI(aq)>>I2(aq)+2NaCl(aq) PbS(s)+4H2O2(aq)>>PbSO4(s)+4H2O(l)
Exam 5 Review Help
Want to verify answers for exam review. Question 1 Consider an electrochemical cell constructed from the following half cells, linked by an external circuit and by a KCl salt bridge: an Al(s) electrode in 1.0 M Al(NO3)3 solution a Pb(s) electrode in 1.0 M Pb(NO3)2 solution What is the balanced overall (net) cell rea
Just a check on my work
Last minute cram for finals tonight. I found these as pertains to my weak areas, so, just to see if I am doing ok, if I am wrong, please correct me-I can probably find where I went wrong! 1. What mass of solid CaO is needed to remove the sulfur dioxide from 1.0 x 106 m3 of air having an SO2 concentration of 1.5 x 10-9 M? Assu
Balancing Example Chemistry Equations
Please give me an explanation of how to balance the following equations. __C2H6 + ___O2 ---> ___CO2 + ____H2O ___C3H6 + ___O2 ---> ___CO2 + ___H2O ___C3H4 + ___O2 ---> ___CO2 + ___H2O ___CH3OH + ___O2---> ___CH2) + ___H2O The solution 1M H2SO4 conducts more current that 1 M HCl? True or false? As the solution
Nickel Oxidation States and Unbalanced Reactions
1. Nickel commonly exists in the +2 and +3 oxidation states. If you are given a 1.47g sample of a mixture of the Ni(II) and Ni(III) salts; determine the % by weight of Ni(II) in the mixture if it required 20.31 mL of .050M KMnO4 to titrate to the end point. The unbalanced reaction is: Ni2+ + MnO4 Ni3+ + Mn2+ (acidi
Effect of aspirin on blood alcohol level
Would it be a good idea for a person to take a couple of aspirin tablets before attending a cocktail party? Explain your reasoning. Response needs to be given in a 200-300 words essay. Include a reference as part of the response.
Oxidation Occurring While Reduction Does Not
Is it possible to have a reaction in which oxidation occurs and reduction does not? Explain and defend your answer.
Quantitative Analysis and Electrochemistry
IX) Given the following information, which of the statements is true? Cu2+(aq) + e- → Cu+(aq) Eº = 0.34V 2 H+(aq) + 2 e- → H2(g) Eº = 0.0V Fe2+(aq) + 2 e- → Fe(s) Eº = - 0.44V Ni(s) → Ni2+(aq) + 2 e- Eº = 0.25V a) Cu+2(aq) is the strongest oxidizing agent b) Cu+2(aq) is the weakest oxi
Ionic Compounds and VSEPR Geometry
Please see the attached file for further details. 9.23 Specify which compound in the following pairs of ionic compounds has the higher lattice energy: (a) KCI or MgO, (b) LiF or LiBr, (c) or NaCl. Explain your choice. See attachment. 9.24 Compare the stability (in the solid state) of the following pairs of compounds:
Nernst Equation with Sodium Chloride
Using the Nernst equation to calculate Ecell at 25 degress C for electrolysis of aqueous sodium chloride if the concentration of chlorine in the brine solution is 4.5 M and the partial pressure of Cl2 is 0.001 atmospheres.
Electrochemistry Problem: Concentration Cells and the Nernst Equation
The cell shown above is a concentration cell. Both cells contain copper solutions and have copper electrodes. The only driving force for this cell is the difference in the concentration of the copper solutions. The system will react to equalize the concentration of the ions in both cells. Calculate ξ, the electromotive forc
5 Electrochemistry Half-Cell Spontaneity Questions
Please see the attached file for complete questions. Question 1: Using and , calculate and for the following reaction: If this is a spontaneous reaction, will it be spontaneous at all temperatures? Question 2: Give the following battery: and and calculate . If you wanted to reduce your of your battery by
Electrochemistry- Cathodes and Anodes
(there are six procedures with the same questions for each procedure) Procedure 1 LAB: Beaker #1: 150 ml CuSO4 Beaker #2: 150 ml SnCl2 Salt Bridge connects the two beakers electrode in CuSO4 is set to copper electrode in SnCl2 is set to tin volt meter reads .4761 Question: 1. Identify the cathode and anode in
Speed of Reaction vs Number of Collisions Required
1.) Compare two chemical reactions, one requiring simultaneous collision of three molecules and the other requiring a collision between two molecules. Assuming all else is equal, which reaction should be faster? Explain your answer. Can you provide an example? 2.) Why do foods cook faster in a pressure cooker relative to an o
Balance the following equation and determine the sum of the coefficients of the products
1) Balance the following equation and determine the sum of the coefficients of the products. ___NO2- + ___MnO4- + ___ H2O à ___ NO3- + ___ MnO2 + ___ OH- A. 3 B. 4 C. 5 D. 6 E. 7 2) Balance the following redox equation and determine the sum of the coefficients of the reactants and products. ___ Br2 + ___
Aluminum : Production and Environmental and Health Concerns
Aluminum is one of the most widely used metals. Aluminum is made by electrolysing aluminum oxide in a solvent called cryolite. Research the electolysis of aluminum and answer the followig questions: a) Identify the products of the anode and cathode half-reactions that occur during the electolysis of aluminum oxide. b) Why is i
