The Lewis Structure depicts the bonding between individual atoms and molecules through illustrating the presence of valence electron pairs. This type of structure/illustration can be drawn for any molecule held together by not only normal covalent bonds, but also coordinate covalent bonds. A normal covalent bond is a chemical bond that involves electron sharing between atoms. A coordinate covalent bond is a bond where the electrons are still shared between atoms; however it differs from a normal covalent bond in that the electron pair originates from just one of the atoms.
For example, the element carbon has the following Lewis structure, where there are 4 valence electrons surrounding it.
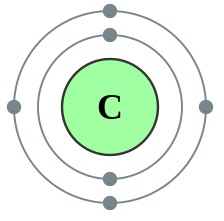
Image Credit: Wikimedia Commons
Methane on the other hand, which is comprised of one carbon and four hydrogen atoms, has the following Lewis Structure:
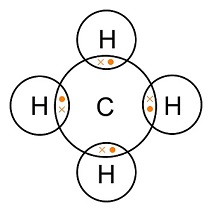
It can be seen that carbon and hydrogen share their electrons to form the four covalent bonds in methane. Thus, by drawing Lewis structures of molecules, no matter how complex they are, it makes it easier to see how the individual atoms are bonded as well as how the electrons interact with each other.
© BrainMass Inc. brainmass.com July 26, 2024, 12:47 am ad1c9bdddf