Stereochemistry is the study of the structures and spatial arrangement of atoms within a molecule. Although the study of stereochemistry falls under Organic Chemistry, it actually extends into the entire spectrum of inorganic, biological, environmental and physical chemistry. Its main focus is on stereoisomers and how to differentiate them. Stereoisomers are a sub-group of compounds that have the same molecular formula and sequence of bonded atoms; however they differ in the three dimensional arrangement of atoms.
There are four types of stereoisomers: enantiomers, diastereomers, E-Z isomers and confomers.
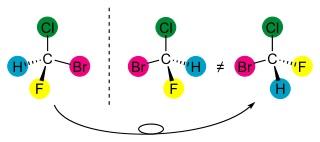
- Enantiomers are two stereoisomers which are mirror images of each other, and are not super-imposable. Thus, the stereogenic center of each enantiomer will have a different chiral configuration.
- Diastereomers are two stereoisomers which are not mirror images of each other. The atoms are arranged very differently in three dimensional space even though it has the same sequence of bonded atoms. This may lead to very different physical and chemical properties between the two diastereomers.
- E-Z isomers refer to isomers with a double bond to restrict the movement of substituents. This can then lead to two different configurations. If the high-priority substituents are on the same side of the double bond, then this is the Z-isomer. If the high-priority substituents are on the opposite sides of the double bond, then this is the E-isomer.
- Confomers refer to molecules with the same structural formula in a different conformation due to bond rotation.
Thus, understanding the many facets of stereochemistry is extremely important in Organic Chemistry, as different stereoisomers will have different properties.
Title Image Credit: Wikimedia Commons
© BrainMass Inc. brainmass.com June 30, 2024, 10:06 am ad1c9bdddf