Cell biochemistry studies chemical reactions and processes that occur in and between biological cells, such information can deepen understanding on the emergent properties of an organism. Cell Biochemistry is highly interdisciplinary topic as it shares important concepts with Molecular Biology and Genetics. Biochemical reactions occur on all scales, from cells, tissues, organs, organ systems and finally the living organism. Cells are studied in terms of their processes, structures, functions, interactions with macromolecules and other cells.
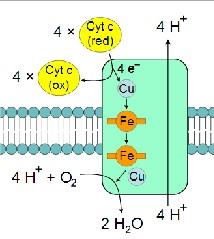
In respiration, the cellular processes convert nutritional energy into chemical energy in the form of ATP for maintenance of internal cell conditions. Biochemical reactions in cellular respiration are separated into different processes of glycolysis, Krebs cycle and electron flow. Understanding cell structure can give further insight to respiration, for example protein structure and synthesis. Cells contain DNA in permutations of base units which are transcripted and translated to produce specific peptides, and polypeptides.
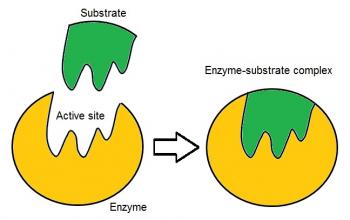
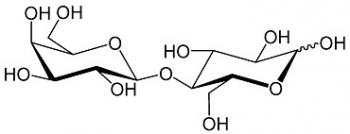
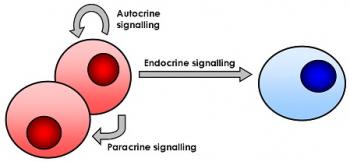
Cell Biochemistry also explores cellular interactions with important macromolecules such as proteins, carbohydrates, and lipids. Enzymes serve as biological catalysts to speed up reactions that break down macromolecules to their constituent units, so cells can use the ATP produced to generate proteins. How cells interact with each other is essential in the functioning of all processes within living organisms. Cell signaling is when a cell sends electrochemical signals to another to produce coordinated responses.
Title Image Credit: Wikimedia Commons
© BrainMass Inc. brainmass.com June 29, 2024, 11:33 pm ad1c9bdddf