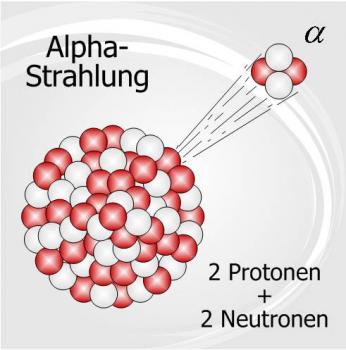
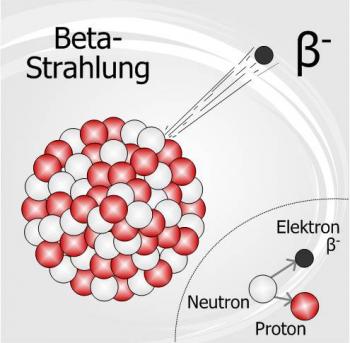
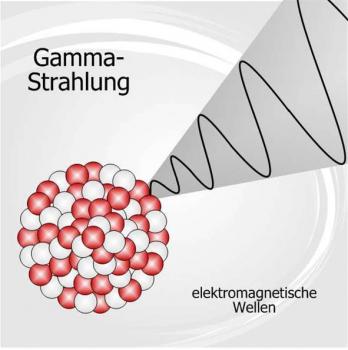
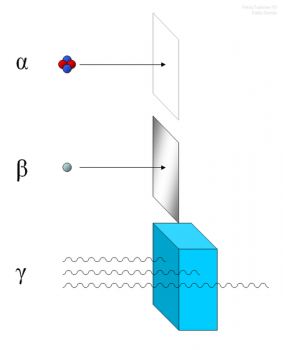
Radioactivity is the spontaneous decomposition of the unstable atomic nuclei to form nuclei with a high stability. The particles and energy released during the decomposition process are called radiation. Radioactivity can occur naturally in the environment or it can be induced in the laboratory.
There are three major types of natural radioactivity, Alpha Radiation, Beta Radiation and Gamma Radiation. These types of radiation can also be induced in a laboratory in order to observe the emission let off. There are other varieties of radioactive decay however they are not as readily studied.
The radioactive decay rates are stated in terms of their half-lives. Different types of radioactivity leads to different decay paths. These different decay paths will convert the nuclei’s into other chemical elements. Radioactive dating occurs by examining the amount of decay product.
© BrainMass Inc. brainmass.com June 30, 2024, 9:20 am ad1c9bdddf