Radiocarbon dating is a technique that utilizes decay of carbon-14 to estimate the age of organic materials. This method is based on the principle that carbon is found in various forms. Through photosynthesis, plants absorb both forms of carbon dioxide in the atmosphere. When an organism dies it contains a ratio of carbon-14 and carbon-12. As the carbon-14 decays it cannot replenish itself and the ratio decreases at a regular rate, its half-life. The measurement of carbon-14 decay provides a measurement of the age of the carbon-based material.
There are fluctuations of carbon-14 and carbon-12 in at atmosphere over periods of time. Scientists use sequencing of tree rings and cave deposits to fine tune and calibrate radiocarbon dating of materials.
The process of radioactive decay gradually decreases the fraction of carbon-14 isotope relative to the other two isotopes of carbon. The half-life of carbon-14 is 5730 ± 40 years. The equation of radioactive decay of carbon-14 is:
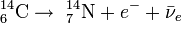
Radiocarbon dating was developed by Willard Libby at the University of Chicago in 1949. Libby estimated that the steady state radioactivity concentration of exchangeable carbon-14 would be about 14 disintegrations per minute per gram. He won the Nobel Prize in chemistry in 1960 for his work.
© BrainMass Inc. brainmass.com July 26, 2024, 12:42 am ad1c9bdddf