A Chiral Molecule is a type of molecule that is non-superposable over its mirror image. On the other hand, achiral molecules are one that are superposable over its mirror image, as in they are identical to their mirror images. Thus, it can be seen that chirality usually stems from some sort of asymmetry in the carbon-based molecule. Consider the following molecule:
Figure 1: (H, R, COOH, NH2)
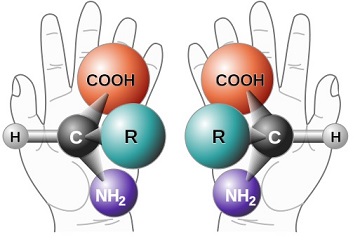
The above amino acid is chiral as if you create its mirror image, it can be seen that these are not identical. Thus, in order to separate non-superposable mirror images, there is an important system to help categorize and identify different chiral molecules. The R- and S- configurations were devised to help categorize molecules based on the priority of the substituents bonded to the center atom. So the first step involves orienting the molecule so that the substituent with the least priority (generally, lowest in atomic/molecular weight) is pointing away from the viewer. Then looking at the remaining three substituents: if the priority decreases in a clockwise fashion, then the molecule will be labeled R; if the priority decreases in a counter-clockwise fashion, then the molecule will be labeled S.
Thus, understanding chirality is extremely important as the concept is heavily involved in a multitude of organic reactions.
© BrainMass Inc. brainmass.com April 19, 2024, 6:29 am ad1c9bdddf